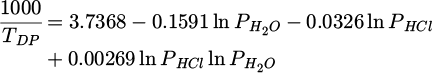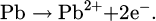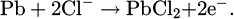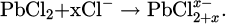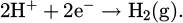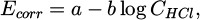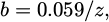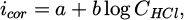| Issue |
Metall. Res. Technol.
Volume 122, Number 5, 2025
|
|
|---|---|---|
| Article Number | 510 | |
| Number of page(s) | 15 | |
| DOI | https://doi.org/10.1051/metal/2025056 | |
| Published online | 30 July 2025 | |
Review
Corrosion of pure lead in HCl at room temperature: a review
Université Paris-Saclay, CEA, Service de recherche en Corrosion et Comportement des Matériaux, 91191, Gif-sur-Yvette, France
* e-mail: florence.lequien@cea.fr
Received:
6
February
2025
Accepted:
6
June
2025
This article provides a comprehensive review of the corrosion behavior of lead in hydrochloric acid (HCl) solutions, a critical area of study due to its industrial relevance and environmental implications. Lead, known for its malleability and resistance to corrosion, exhibits complex degradation mechanisms when exposed to acidic environments. The research examines the electrochemical processes involved, including the dissolution of lead and the formation of soluble lead species, particularly focusing on the transition from lead carbonate to lead chloride. Various factors influencing corrosion rates, such as HCl concentration, temperature, and environmental conditions, are discussed. Additionally, this review highlights recent advancements in corrosion inhibition strategies aimed at mitigating lead degradation. Understanding these mechanisms is essential for extending the lifespan of lead-based materials in industrial applications and addressing environmental concerns associated with lead exposure.
Key words: Corrosion / HCl / lead / room temperature
© EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
Lead, an element of significant historical and industrial importance, is one of the 36 elements constituting the Earth’s crust. It is a chemical element classified within the carbon group, with the symbol Pb and atomic number 82. The name “lead” is derived from the Latin term “plumbum,” which translates to “liquid silver.” This bluish-gray metal is characterized by its brilliance, malleability, ductility, and low melting point, making it easy to work with in various applications. Lead is one of the heaviest metals widely utilized in metallurgical activities since antiquity and rediscovered during the Industrial Revolution [1]. Lead exhibits several notable properties that contribute to its extensive use across various industries. It has a relatively low melting point of approximately 327.5 °C and a boiling point of about 1,740 °C. Its density is high, around 11.34 g/cm3 at room temperature, which makes it advantageous in applications requiring weight and stability. Despite its beneficial properties, lead poses significant health risks due to its toxicity [2]. It has been classified as a probable human carcinogen and is known to have mutagenic and reproductive toxicity effects [3]. Therefore, while lead remains an essential material in many applications [4–6], including batteries, radiation shielding, plumbing fixtures, and solder, it is critical to manage its use carefully to mitigate health risks associated with exposure [7], and to be able to recycle it [8]. Lead is also recognized for its excellent resistance to corrosion, which allows it to maintain integrity in various environmental conditions. However lead exhibits complex corrosion behavior that varies significantly depending on the environment [9–11]. While it demonstrates high resistance to certain acids like acetic [12], sulfuric [13], chromic, and phosphoric due to the formation of protective layers, its behavior in hydrochloric acid HCl presents unique challenges and opportunities for study. The corrosion of lead in HCl is a critical area of research with far-reaching implications for various industries and environmental concerns. When exposed to acidic environments, particularly HCl, lead undergoes significant corrosion, leading to material degradation and potential environmental hazards. The study of lead corrosion in HCl is paramount for several reasons:
Industrial relevance: Lead is utilized in chemical processing equipment [14], battery manufacturing, and other applications where exposure to HCl is possible.
Environmental impact: The release of lead ions due to corrosion poses significant environmental and health risks, necessitating a thorough understanding of the corrosion process [15].
Material performance: Comprehending corrosion mechanisms is crucial for predicting and extending the lifespan of lead-based materials in acidic environments.
Corrosion protection: Knowledge of the corrosion process is essential for developing effective protection strategies and corrosion inhibitors.
Archaeological artifacts: HCl is often used as a cleaning agent for archaeological artifacts, making it essential to understand its potential impact on lead-based historical objects [16].
Despite environmental concerns and stricter regulations leading to the search for alternatives, lead still finds applications in various industries. The lead market size has grown rapidly in recent years. It will grow from $19.16 billion in 2024 to $21.38 billion in 2025 at a compound annual growth rate of 11.6% [17]. Its use in sealing underground power and communication cables, lead-tin solders, electrode plates in lead-acid storage batteries, and chemical processing underscores the ongoing need to understand and mitigate its corrosion behavior, particularly in acidic environments like HCl.
This review aims to provide a comprehensive overview of the current understanding of lead corrosion in HCl solutions. We will examine the fundamental mechanisms of corrosion, factors influencing the corrosion rate, methods of study, and the modeling of lead corrosion. Additionally, we will discuss recent advancements in corrosion inhibition.
2 Atmospheric corrosion of lead
2.1 Overview
The formation of corrosion products in atmospheric conditions plays a significant role in protecting metals by creating thin layers that limit further corrosion. The nature of these products varies depending on the exposure conditions and the presence of pollutants [11,18]. The corrosion of lead in atmospheric conditions has been extensively studied since the late 19th century, with early contributions such as those by Gaines [19]. Common corrosion products of lead include oxides, carbonates, and, in the presence of specific pollutants, chlorides, sulfides, and organic compounds like acetates and formates. Lead (II) oxide (PbO), often in the form of litharge, typically forms first in clean environments and is around 3 to 6 nm thick [20,21]. Removing these corrosion products from historical lead artifacts is challenging, particularly in hydrochloric acid (HCl) solutions [18].
Different environments distinctly influence the corrosion mechanisms of lead, with urban and rural settings producing notably different corrosion products due to varying pollutant levels. In urban environments, the presence of sulfur dioxide (SO2) from industrial emissions accelerates the formation of lead sulfate (PbSO4), significantly increasing corrosion rates [22]. These pollutants not only promote the formation of corrosive products but also disrupt the protective oxide layers that typically form on lead surfaces, rendering the metal more susceptible to degradation. Conversely, in rural environments, where pollution levels are lower, lead predominantly reacts with carbon dioxide (CO2) and humidity to form more stable carbonate compounds such as cerussite (PbCO3) and hydrocerussite (Pb3(CO3)2(OH)2) [23]. This difference underscores the impact of specific atmospheric constituents on the corrosion process, with carbonates being the primary corrosion products in less polluted areas. Moisture is a crucial factor in the corrosion of lead, as it facilitates electrochemical reactions on the metal’s surface. The combination of humidity and pollutants creates an environment conducive to rapid corrosion, especially in outdoor settings where lead artifacts are continuously exposed to atmospheric conditions. Sulfur-rich environments further complicate this process, with the formation of lead sulfides and sulfates, particularly anglesite (PbSO4), as common corrosion products. Indoor environments, such as museums, generally exhibit slower corrosion rates due to controlled atmospheric conditions. However, these settings are not entirely immune to corrosion, particularly from volatile organic compounds (VOCs). Lead shows heightened susceptibility to VOCs, especially volatile organic acids like acetic acid, which can lead to the formation of lead acetate and formate, thus accelerating corrosion [24]. Elevated VOC levels necessitate careful monitoring in areas where lead artifacts are stored or displayed to mitigate corrosion risks [25].
The classification of atmospheric environments according to the ISO 12944-2 standard provides a framework to understand the varying corrosivity levels, from very low (C1) to very high (C5), which directly impacts the corrosion rates of lead [26]. As shown in Table 1, lead corrosion rates range from 0.05 μm/yr in controlled indoor environments to 14 μm/yr or more in highly corrosive outdoor environments [27,28].
Lead corrosion rates and mechanisms in outdoor and indoor settings have been compared, offering valuable insights into the environmental factors that influence lead degradation [28]. Quantitative data on corrosion rates, enabling a more precise understanding of lead’s long-term behavior in diverse atmospheric environments is presented in Table 2. This research is particularly noteworthy for its practical implications in heritage conservation, as it provides crucial information for preserving lead artifacts in museums and historical sites. Lead in urban environments, such as on the rooftops of cathedrals, undergoes accelerated corrosion due to industrial pollutants, leading to significant material loss over time [19]. In contrast, lead artifacts in museums show slower corrosion rates, but specific VOCs such as formic acid can lead to localized deterioration, necessitating precise environmental control [25].
2.2 Atmospheric corrosion in an environment polluted by HCl
Hydrogen chloride HCl(g) can be present in the environment due to industrial emissions, volcanic activity, or marine aerosols [29,30], and it can exacerbate corrosion processes by lowering the dew point temperature [31], thus increasing the likelihood of moisture condensation on metal surfaces and promoting electrochemical reactions. Indeed, an humid air polluted by HCl consists of a gas that is a strong acid that completely ionizes in aqueous solution. In the presence of water, HCl(g) tends to be trapped by the liquid. If the water content in the air is sufficiently high, a condensation phenomenon may occur. In the absence of HCl(g), the conditions (in terms of temperature T, pressure P, and relative humidity HR) for the appearance of these droplets can be predicted thermodynamically. However, the values of the parameters leading to condensation are modified by the presence of HCl(g). To evaluate the dew point temperature in the presence of HCl, Kiang established a relationship between the partial pressures of water and hydrogen chloride and the dew point temperature [31]:
where TDP is the dew point temperature in Kelvin, and PH2 O and PHCl are the partial pressures in mmHg. Experimentally, the partial pressures were measured by Fritz and Fuget [32] above dilute acid solutions and by Zeisberg [33]. For low concentrations of HCl(g), the dew point temperature of the gas mixture is lower than the dew point temperature of pure water. The pollutant HCl appears to have little impact in relation to water. However, for high pressures of HCl (a few tens of ppm), the dew point temperature of the mixture can become higher than that of pure water. For example, if the partial pressure of water is 1000 Pa, the dew point temperature of pure water is 6.96 °C. A partial pressure of HCl of 10 ppm is sufficient to yield a dew point temperature exceeding that of pure water by 8.6 °C. The fact that the dew point temperature of an H2O/HCl mixture is higher than that of pure water is consistent with the notion that a pollutant increases the dew point temperature [22].
The corrosion mechanism of lead in hydrochloric acid solutions involves several interrelated processes that contribute to its degradation (Tab. 3). The corrosion mechanism of lead in humid and Hcl polluted environement is significantly influenced by the transition from lead carbonate PbCO3 to lead chloride PbCl2 [23]. In the presence of HCl, the acidic environment facilitates the dissolution of PbCO3, leading to the formation of soluble PbCl2: the solubility product (Ksp) of PbCO3 is much lower than that of PbCl2 [35], indicating that PbCO3 tends to precipitate and form solid deposits more readily than PbCl2. This means that in acidic environments like hydrochloric acid, the presence of chloride ions can lead to the dissolution of lead carbonate and the formation of soluble lead chloride, which can significantly influence the corrosion behavior of lead. The formation of PbCl2 can also lead to the establishment of a passivating layer that may inhibit further corrosion; however, this layer’s stability can be compromised under various conditions, such as changes in chloride ion concentration or pH levels. The dynamics of this corrosion process are complex, with the dissolution of lead being closely linked to the concentration of HCl, the temperature, the electrolyte size. The complexity of lead oxidation and mineral formation depends significantly on environmental conditions, highlighting the dynamic surface chemistry of lead when exposed to different atmospheric and aqueous environments [20], and this explains the numerous studies dedicated to the treatment of lead artifacts [16,36]. The main aspects of which are summarized in Figure 1. The corrosion mechanism of lead in hydrochloric acid solutions is indeed complex, involving multiple processes and intermediate products. In addition to the formation of PbCl2, hydrated corrosion products can also form, creating more intermediate compounds. For example Pb3O2Cl2, known as mendipite, is an example of a more complex corrosion product that can form in the presence of both oxygen and chloride ions [23]. However, thermodynamic data for such intermediate compounds are often limited or unavailable, making it challenging to fully model their behavior in corrosion processes (Tab. 3). The formation of these hydrated and complex corrosion products can significantly influence the corrosion kinetics and the nature of the protective layers formed on the lead surface.
Ongoing research continues to refine methods for quantifying atmospheric corrosivity towards lead. A recent study has highlighted that using color change values after a 3-month exposure period is more appropriate for assessing indoor air aggressiveness on lead. This approach proves more relevant than annual exposure, which disrupts the linear relationship between color change and corrosion rate. These findings demonstrate that scientists are still actively working on improving techniques to accurately quantify atmospheric corrosivity on lead, seeking more reliable indicators and optimal exposure periods [27].
Lead can indeed undergo pitting corrosion. This is due to the ability of halide ions to attack surface films. Pitting occurs under conditions of partial passivity or cavitation, which is the formation and collapse of gas bubbles at a liquid/metal interface [13]. However, to limit its impact, the physical characteristics of the material are adjusted, such as a high material thickness [22]. In 0.3 MHCl, Salghi shows that Pb doesn’t suffer from localized attack [37]. In HCl, pure tin exhibits excellent resistance to pitting corrosion, remaining largely unaffected by the aggressive environment. However, when it comes to lead-tin alloys (Pb-Sn), the situation changes significantly. The corrosion sensitivity of these alloys is notably increased compared to pure tin, making them more susceptible to pitting. This enhanced vulnerability can be attributed to the presence of lead in the alloy, which alters the electrochemical behavior and protective characteristics of tin. As a result, while pure tin remains stable in HCl, lead-tin alloys are prone to localized corrosion [38].
Balance equation of the reactions involved in the corrosion mechanism of lead exposed to a humid and polluted by HCl environment.
 |
Fig. 1 Schematic representation of lead corrosion processes in HCl environment. |
3 Corrosion in HCl solution
According to Pourbaix [39,40], the stability domains of lead and its compounds indicate that lead behaves as a relatively noble metal at pH values greater than 5, leading to the formation of lead oxide (PbO). However, at lower pH levels, particularly in acidic environments like HCl, lead tends to dissolve into Pb2+ species. This dissolution process is significant as it results in an increase in the concentration of lead ions in solution, which poses environmental risks due to potential contamination of water sources. In acidic conditions, PbO exhibits high solubility [41], further contributing to the generation of Pb2+ ions. Additionally, lead demonstrates passivation through the formation of lead dioxide (PbO2) across a broad pH range; however, this occurs only at higher potential values. The relationship between pH and the stability of lead species is crucial for understanding corrosion mechanisms and environmental impacts.
3.1 Dissolution study of Pb in HCl
Lead readily reacts with hydrochloric acid, resulting in its dissolution and the formation of soluble lead species. As the hydrochloric acid concentration rises, the chloride ion concentration correspondingly increases. This elevated chloride ion concentration has been shown to influence the formation of complexes with metal ions in solution. Specifically, as the total concentration increases, there is a notable trend: the proportion of metal ions forming neutral or negatively charged complexes grows. This shift in complex formation is directly related to the increased availability of chloride ions in solution [34]. The dissolution process is complex and strongly influenced by the concentration of HCl. In dilute solutions HCl, lead(II) chloride PbCl2 exhibits low solubility due to the common ion effect, which suppresses dissociation according to Le Chatelier’s principle. However, as the concentration of HCl increases, the solubility of lead improves significantly due to the formation of soluble complex ions (Tab. 4). The shift in equilibrium toward these soluble complexes, driven by increasing chloride ion concentration, leads to enhanced lead dissolution in concentrated HCl.
The kinetics of lead dissolution in HCl revealed that the steps controlling the dissolution rate are initially dominated by surface chemical reactions, followed by diffusion processes. The activation energies for these steps were measured at 60.94 kJ/mol for the initial chemical reaction and 28.24 kJ/mol for liquid-phase diffusion [45]. These values suggest that the dissolution process is primarily controlled by chemical reactions at the surface, particularly in the early stages. HCl is generally considered among the most effective acids for the dissolution of lead from oxidized ores, with an increase in the acid concentration significantly enhancing solubility due to the formation of soluble lead complexes, such as PbCl2 and higher chloride complexes [46]. Qualitative corrosion data provide useful guidelines, but laboratory test conditions can differ significantly, leading to variations in the results. Nevertheless, lead is generally classified as category C, indicating a corrosion rate typically between 500 and 1200 μm/yr in most industrial cases. This classification suggests that lead can be used in certain applications where its corrosion and long-term degradation are acceptable. The corrosion rates of chemical lead in HCl acid can be accelerated or retarded by the presence of other elements in solution. With H2SO4 [42], the lead dissolution is retarded: from 610 μm/yr in 1% HCl to 130 μm/yr in 1% HCl+ 9% H2SO4, whereas it’s accelerated with FeCl3 (Tab. 4).
Many studies have focused not on the dissolution of pure lead but rather on its alloys, particularly those with tin. As early as 1932 [47], a study reported the challenges of titration of tin while lead dissolves quickly in HCl. This research highlighted the complexities involved in the analysis of lead-tin alloys, where the differential dissolution rates of the component metals posed significant analytical challenges. Rapid dissolution of lead in hydrochloric acid contrasted sharply with the difficulties encountered in accurately determining the tin content.
Comparison of pure lead dissolution kinetics in solutions with HCl.
3.2 Electrochemical study
The first electrochemical studies of lead in HCl date back to the 1970s and were conducted primarily by Barradas and his coauthors [48–52]. This increased focus on lead and lead chloride corrosion studies is indeed linked to the development of Pb/PbCl2 electrodes. During this period, researchers were investigating the electrochemical properties of these electrodes for various applications, including environmental monitoring and corrosion studies. The Pb/PbCl2 electrode system is notable for its stability and non-polarizing characteristics, making it suitable for use in electrochemical measurements. This electrode configuration relies on the equilibrium established between lead and its chloride form, which is crucial for understanding corrosion processes in acidic environments, particularly those involving hydrochloric acid.
Electrochemical studies investigate both the interaction between a lead electrode and an electrolyte composed of a hydrochloric acid solution, as well as the electrochemical behavior of lead and its compounds, notably PbCl2.
The corrosion of lead in an acidic environment such as hydrochloric acid is a complex electrochemical process. Metallic lead (Pb) reacts with chloride ions (Cl−) present in hydrochloric acid, forming lead ions (Pb2+) and releasing electrons according to the following reaction:
This leads to a substantial rise in measurable current, occasionally featuring a visible pre-peak. This pre-peak is attributed to either the presence of sparse small crystals on the electrode surface [51] or a PbCl2 monolayer [48] that can undergo localized dissolution as the current decreases at the end of the peak, resulting in the formation of defect sites.
Near the electrode surface, the solution becomes concentrated with lead ions, creating a supersaturated solution close to the electrode surface. This allows for the formation of nuclei that quickly develop into crystals covering the surface [51,53]:
The formation of PbCl2 is a reversible process controlled by the diffusion of chloride ions (Cl–) toward the electrode surface [54]. The growth of the layer requires the formation of Pb2+ ions, which originate from both the electrochemical process and the fact that the layer is sometimes described as a bilayer, with an inner layer that can evolve to become porous or develop a complicated morphology [48,51]. Various surface analyses, such as Scanning Electron Microscopy (SEM) and X-Ray Diffraction (XRD), demonstrate that the process is complex, involving numerous reaction intermediates of the type 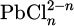 with an evolution from ion state to solid phase [48,51].
with an evolution from ion state to solid phase [48,51].
Additionally, the morphology of the PbCl2 layers formed is highly dependent on the applied voltammetric conditions [54].
Beyond the activation peak, the current increases slightly, which can be explained by the breakdown of the film due to a dissolution reaction [49,53] of the general form:
In acidic media, the reduction of hydrogen ions (H+) to hydrogen gas (H2) is typically considered the primary cathodic reaction:
This reaction contributes to the continuous acidification of the medium. However, electrochemical spectra reveal that the cathodic process is more complex, consisting of two distinct peaks. These peaks are attributed to the reduction of Pb and PbCl2 [50]. This dual-peak nature of the cathodic process indicates that the electrochemical behavior of lead in acidic chloride solutions involves multiple reduction pathways, beyond the simple hydrogen evolution reaction. In oxygenated solution, the small cathodic peak can be attributed to the reduction of some hydrolyzed species, such as PbOHCl, formed as a result of localized pH changes at the electrode surface during electrolysis [52]. Although many authors take precautions to conduct studies under nitrogen, Barradas et al. hypothesize that the PbCl2 layer is not significantly affected by traces of dissolved oxygen or the presence of OH– [51], as PbO does not form in acidic pH (pH>3) under low O2 concentration [52].
3.2.1 Effect of the HCl concentration
The influence of hydrochloric acid concentration on the electrochemical behavior of lead electrodes has been extensively studied. Within a concentration range of 0.01 to 2.00 M, linear sweep voltammograms indicate that at HCl concentrations above 0.20 M, a passive region forms, characterized by a relatively low residual current that decreases with increasing acid concentration [55].
This decrease is attributed to the thickening of the passive layer of PbCl2 or to surface blockage by larger PbCl2 crystals. Additionally, it was observed that the potential corresponding to the anodic peak current shifts to more negative values with increasing HCl concentration, suggesting that the formation of the passive layer is facilitated. The height of the anodic peak IA decreases when the concentration of HCl is increased. The relation between log CHCl and log IA is not linear with an abrupt change in the slope of the line for a 0.5 M HCl concentration. This change in the slope seems to indicate a transition in the electrochemical behavior of the lead anode: at low concentrations of HCl (up to about 0.5 M) the growth of the surface layer of PbCl2 may be limited and controlled by diffusion of the Cl– anions. This suggests that the rate of formation of PbCl2 is lower than the rate of anodic dissolution of lead, indicating that passivation of the lead is delayed. At higher concentrations of HCl (above 0.5 M) it seems probable that there is no limitation by diffusion of the Cl– ions and that the layer of PbCl2 is formed more readily [55]. A similar transition in the behavior of the lead anode in HCl solutions was observed at concentrations around 0.7 M HCl, where the solubility of PbCl2 in aqueous chloride solutions reaches a minimum [50]. An optimal HCl concentration of 1.00 M was identified for achieving a thick and porous passive layer on the lead electrode, which is essential for electrochemical applications [54,55]. Potentiodynamic analysis also revealed that the corrosion potential (Ecorr) shifts to more negative values as acid concentration increases, establishing a linear relationship between Ecorr and log CHCl :
where a and b are constants. Some studies attempted to determine these parameters. a corresponds to the Pb electrode potential. It is estimate in 1 M HCl at −0.370 VSCE [56] which is in accordance with the standard electrode potential (Tab. 5) [34] or −0.508 VSCE [46]. This second value seems more coherent with the Ecorr that is determined graphically as for example Lateef [61] which found a Ecorr value of -0.554 VSCE in 1 M HCl. b is generally express as:
where z refers to the number of electrons involved in the anodic or cathodic reaction process. A slope is generally of −30 mV/decade and gives z = 2 [49,56–62]. The corrosion current density (icor) is directly influenced by the concentration of hydrochloric acid in the solution, like the corrosion potential. As the concentration of HCl increases, the availability of hydrogen ions (H+) also increases, leading to a higher rate of electrochemical reactions at the metal surface. This results in an increase in the corrosion current density, which can be quantitatively described using Tafel equations. For instance, the relationship can be expressed as:
where icor is the current density, CHCl is the HCl concentration, and a and b are constants that depend on specific conditions, including HCl concentration. For concentrations between 1 × 10−3 and 1 × 10−1 M HCl at room temperature, a is 0.22 mA·cm−2 and b equals 60 × 10−3 mA·cm−2· decade−1 [46].
3.2.2 Effect of rotation electrode [50]
The effect of rotation on electrodes, particularly rotating disk electrodes (RDEs), is significant in electrochemical studies. Rotation enhances mass transport by creating a constant flux of analyze to the electrode surface, leading to steady-state currents controlled by solution flow rather than diffusion. The rotating disk generates a hydrodynamic boundary layer, where centrifugal force flings solution away from the center, replaced by solution flowing up from the bulk. This results in higher current density at the edges compared to the center, slightly increasing the overall disk current above predictions made by the Levich equation. The Levich equation, fundamental to RDE theory, relates the limiting current to the angular velocity of the electrode, providing a means to determine diffusion coefficients and study reaction mechanisms [63]. The rotation speed also affects the thickness of the diffusion layer, with higher speeds resulting in thinner layers, which can significantly impact kinetic measurements. RDEs are particularly useful for studying mass transfer-limited and electron transferlimited reactions, allowing researchers to distinguish between these processes. The impact of a rotating disc electrode have been investigated by Barradas et al. in [63]. With a rotating disc electrode, an increase in current is observed compared to a stationary disc, for HCl concentrations above 0.4 M. Peak currents were generally proportional to ω1/2 (where ω is angular velocity), but behavior in the reactivation region is more complex. The ratio of cathodic charge and anodic charge shows dependence on rotation speed, with values nearing unity at zero rotational speed and a maximum at high sweep rates, suggesting minimal dissolution under certain conditions. This behavior aligns with solubility predictions, indicating least dissolution when mass transport is minimized. For smaller HCl concentrations, surface growth becomes increasingly difficult as rotation is stopped, indicating a dependence on Cl– ion concentration and hydrodynamic conditions. Cathodic peaks increase in size when electrode rotation is stopped, suggesting possible concurrent reactions of surface layer reduction and reduction of soluble species. Notably, electrode reactivation upon rotation in low concentrations is similar to that observed in higher concentrations, highlighting the complex interplay between electrode kinetics, mass transport, and solution composition in these electrochemical systems. More generally, rotation of the electrode increases the amount of dissolution, enhances the transport of Cl– ions to the electrode surface, and leads to more rapid passivation. At Cl– ion concentrations greater than 1.0 M, there is no limitation by diffusion of Cl– to the surface of the electrode, passivation of the electrode was easily attained.
3.2.3 Cyclic Voltammetry [48,53,51]
Cyclic voltammetry (CV) is a powerful electrochemical technique used to study the behavior of electrodes and electroactive species in solution. In the context of lead electrodes in HCl solutions, this method has provided valuable insights into the passivation process and the formation of protective layers on the electrode surface. When applied to lead electrodes in HCl solutions, cyclic voltammetry reveals a characteristic behavior: as the potential is swept in the positive direction, an anodic current is observed, corresponding to the oxidation of lead and the formation of a PbCl2 layer. This process leads to the passivation of the electrode, where a protective film forms on the surface, reducing further corrosion. Moreover, in 1.0 M HCl, the anodic scan reveals a pre-peak at approximately −520 mV, followed by the main oxidation peak at −455 mV [53]. SEM analysis confirms the formation of a PbCl2 layer [51]. The peak separation of about 90 mV indicates the irreversible nature of the surface reaction [51]. The ratio of cathodic to anodic currents depends on the sweep potential, reflecting changes in the passive layer formation and thickening [48]. The presence of oxoanions, such as sulfate and phosphate, can significantly influence this behavior by either stabilizing or destabilizing the protective PbCl2 layer. Repeated cycling demonstrates a reversible process of PbCl2 formation and dissolution, despite the progressive increase in PbCl2 thickness [48]. Holding time at specific potentials plays a crucial role in the thickening of the PbCl2 layer, particularly just beyond the activation potential [48]. Increasing sweep rates lead to a consistent increase in both anodic and cathodic currents, maintaining a near-unity ratio, suggesting diffusion-controlled PbCl2 formation [48]. This dynamic interplay between lead corrosion and oxoanions underscores the complexity of passivation mechanisms in acidic environments. The reversible nature of the PbCl2 formation/dissolution process, coupled with the progressive thickening of the passive layer, underscores the dynamic character of the lead electrode surface during cyclic voltammetry experiments.
3.2.4 Repeated cycle on cyclic voltammetry
The effect of repeated cycles on the electrochemical behavior of lead electrodes in a 1.00 M HCl solution has been studied using cyclic voltammetry at a sweep rate of 1.67 mV/s and a potential of −450 mV [54]. During repeated cycles, the lead electrode exhibits distinct anodic and cathodic peaks corresponding to the oxidation and reduction reactions of lead. In the initial cycles, both anodic current (jap) and cathodic current (jcp) increase with the number of cycles, while the ratio jcp/jap remains close to unity. This suggests a gradual increase in the effective electrode surface area due to the formation of spongy lead during the negative cycle, facilitating greater surface area for the formation of the PbCl2 passivating layer. Despite this increase in currents, the positions of anodic and cathodic potentials do not change significantly, and the peak separation remains low, indicating that the process remains reversible even after 20 cycles. In a second series of experiments where the potential was held at 400 mV and the sweep rate increased to 50 mV/s, voltammograms showed broader peaks and a continuous increase in currents up to 30 cycles. It was noted that in the first cycle, jcp exceeded jap with a ratio reaching 2.1, likely due to an increased thickness of the PbCl2 layer; however, from the second cycle onward, this ratio gradually decreased to a stable value around 1.2 after 30 cycles. Additionally, peak potentials showed a gradual shift with repeated cycles, resulting in a corresponding increase in peak separation. These results suggest that uncompensated resistance in the solution becomes more significant at higher sweep rates, affecting measured potentials. These observations highlight the importance of repeated cycling for optimizing both the formation and integrity of the passivating layer on lead electrodes under acidic conditions. Moreover, the number of cycles influences the formation of cathodic peaks and the growth of passivating layers [50]. Progressive cycling experiments show that the cathodic peaks observed during reverse sweeps are primarily formed during this inversion, emphasizing the importance of temporal dynamics in the corrosion process. The study [54] also explored the electrochemical behavior of lead electrodes in HCl solutions ranging from 0.01 to 2.00 M, emphasizing the formation of a thin yet effective PbCl2 layer on the electrode surface. Using an appropriate concentration of HCl (1.00 M), combined with controlled sweep field and sweep rate, enhances the stability and thickness of this layer. Furthermore, maintaining potential for a period (particularly just beyond anodic potential (Eap)) promotes thickening of this layer. Cyclic voltammetry reveals that PbCl2 formed during positive cycling is reduced to spongy lead during negative cycling, accompanied by an increase in surface roughness. Repeated cycling leads to progressive thickening of both spongy lead and PbCl2, with the latter exhibiting resistance to rupture or degradation. The results indicate that this electrochemical method is an effective pathway for preparing active PbCl2 material for use as a cathode alongside magnesium alloy anodes in marine batteries.
3.2.5 Impact of temperature
Temperature plays a crucial role in the electrochemical behavior of lead electrodes in HCl solutions. As temperature increases, the kinetics of electrochemical reactions generally accelerate, leading to enhanced corrosion rates. Specifically, for every 10°C rise in temperature, the rate of electrochemical reactions can approximately double, which significantly affects the stability and integrity of protective layers like lead chloride (PbCl2) formed during cyclic voltammetry experiments. As the temperature of a 0.1 M HCl solution is raised from 15°C to 55°C, both the dissolution potential of lead (Edissol) and the reactivation potential (Ereactivation) shift negatively, while the duration of the dissolution arrest increases from 1.35 to 6.2 min [56]. This behavior suggests that higher temperature not only increase the dissolution rates of lead but also destabilize passivating films, making them more susceptible to localized corrosion due to aggressive ions present in HCl solutions. Moreover, the study found a linear correlation between temperature and both Edissol and Ereactivation, with shifts of 4.3 mV per degree and 10.8 mV per degree, respectively. This indicates that elevated temperatures enhance the solubility of corrosion products such as PbCl2, further contributing to increased corrosion rates. Barradas’ study complements these findings by demonstrating that the anodic behavior of cycled lead electrodes is influenced by temperature through mechanisms involving nucleation and growth processes. The mode of passivation is characterized by the nucleation and growth of threedimensional PbO nuclei, which slowly dissolve into solution. At extreme anodic potentials or low temperatures, passivation likely occurs via progressive two-dimensional growth under diffusion control. This highlights that at all investigated potentials, the mass transport of PbCl2– species away from the electrode surface is critical in determining electrode kinetics [64]. Conversely, lower temperatures tend to slow down reaction kinetics, potentially enhancing the longevity of protective layers but may also reduce the overall efficiency of electrochemical processes. Studies have shown that maintaining optimal temperature ranges is essential for reliable electrochemical measurements and for understanding the mechanisms underlying lead corrosion in acidic environments. Moreover, temperature variations can influence the solubility of PbCl2 and other corrosion products, thereby affecting their deposition and stability on the electrode surface. In particular, it has been noted that higher temperatures can disrupt protective layers formed on lead surfaces, making them more susceptible to aggressive ions present in HCl solutions [65]. This interplay between temperature and electrochemical behavior underscores the necessity for controlled experimental conditions when investigating lead corrosion in HCl solutions.
4 Inhibition
The inhibition of lead corrosion in aggressive environments such as hydrochloric acid is a critical aspect of corrosion protection. It is essential to ensure the longevity of lead-based components, mitigate potential failures, and comply with environmental regulations. Furthermore, effective corrosion protection strategies facilitate material recycling and disposal at the end of their life-cycle. However there is limited research on the inhibition of lead corrosion in hydrochloric acid.
Among the available methods, the use of corrosion inhibitors has emerged as a cost-effective and efficient approach to mitigating corrosion. These inhibitors, when introduced at low concentrations into corrosive environments, significantly decelerate or halt the corrosion process.
The mechanisms of corrosion inhibition are generally categorized into two principal pathways (Fig. 2): (i) modification of the corrosive medium, often achieved through interaction with corrosive species, and (ii) direct interaction with the metal surface to form a protective barrier against corrosive agents [66,67]. The efficiency of these inhibitors is influenced by several factors, including the type of metal, the chemical composition of the corrosive medium, and the molecular characteristics of the inhibitors. Current research efforts are increasingly focused on developing environmentally benign inhibitors with enhanced performance across a broad spectrum of applications.
Organic compounds containing heteroatoms such as nitrogen, oxygen, and sulfur have demonstrated considerable efficacy as corrosion inhibitors for lead in HCl environments [68]. These compounds typically function by forming protective films on the lead surface, thereby reducing the corrosion rate. Inhibition efficiency has been shown to correlate with molecular attributes such as electron density, functional groups, and structural configuration. The adsorption behavior of these inhibitors on lead surfaces frequently follows adsorption isotherms, including the Langmuir and Temkin models. Both physisorption and chemisorption are implicated in the inhibition mechanism, depending on the specific inhibitor and the prevailing experimental conditions. Experimental studies have demonstrated the effectiveness of N-phenylcinnamimide and its derivatives as corrosion inhibitors for lead in HCl solutions. Weight-loss experiments revealed that these compounds significantly reduce the corrosion rate of lead. Notably, the inhibition efficiency was found to increase with both immersion time and inhibitor concentration [69]. The protective effect is attributed to the adsorption of these compounds on the lead surface, which follows Langmuir and Temkin isotherms, indicating the formation of a stable protective film. The addition of nitrogen-containing compounds like phenylendiamene (PDA) affects these anodic and cathodic mechanisms by increasing hydrogen overvoltage on the corroding metal [43]. This action can enhance hydrogen evolution reactions while also serving as mixed-type inhibitors for metals like zinc and iron or accelerators for other metals like lead due to their ability to modify activation energies associated with corrosion processes [46]. In addition to organic inhibitors, amino acids have been identified as promising candidates for lead corrosion inhibition. Glutamic acid, in particular, exhibits significant inhibitory efficiency across a range of pH values (2, 7, and 12). Among several amino acids studied, glutamic acid demonstrates superior performance in reducing lead corrosion [70]. The inhibition mechanism involves the adsorption of positively charged amino groups onto the lead surface, effectively blocking active corrosion sites and limiting the exposure of lead to corrosive ions.
Moreover, studies employing cyclic voltammetry have shown that increasing the concentration of inhibitors results in a decrease in anodic and cathodic peak currents, indicative of enhanced inhibitor adsorption on the lead surface [71]. Anodic polarization analyses further reveal that the charge transfer resistance increases with inhibitor concentration, signifying improved protective effects.
Emerging trends in corrosion inhibition research emphasize the development of green inhibitors derived from natural sources and the design of synergistic formulations to maximize efficiency [72]. Natural extracts contain a diverse array of active elements that can interact with metal surfaces to form protective films. These films act as barriers against corrosive agents, thereby preventing further degradation of the metal. The abundance of these active compounds in natural sources makes them an excellent foundation for developing new green corrosion inhibitors. In addition to natural extracts, ionic liquids have emerged as promising green corrosion inhibitors. Their unique synthetic pathways and properties position them as innovative solutions for corrosion protection. The mechanisms by which these green inhibitors operate—particularly their adsorption processes—are crucial to understanding their protective capabilities across various fluids and metal alloys.
Various natural and synthetic inhibitors that effectively reduce lead corrosion rates in HCl have been study in the context of patrimony protection, showcasing their mechanisms of action, such as adsorption onto metal surfaces to form protective films. These inhibitors not only enhance the longevity of lead materials but also align with environmental regulations aimed at reducing hazardous substances [73]. The sodium decanoate is used as a corrosion inhibitor. The mechanism of protective film formation involves an initial oxidation step of the substrate. Once the critical concentration of lead ions is reached at the metal/solution interface, their combination with the carboxylate causes the precipitation of a hydrophobic and covering metallic soap, with the formula Pb(Cn)2 where Cn = CH3 (CH2)n-2COO-. After a simple immersion in a sodium decanoate solution, three-dimensional layers of crystallized lead carboxylates, with the formula (CH3(CH2)n−2-COO)2Pb, have been identified on the metal surface, protecting the material [73].
Phosphates serve as effective mineral inhibitors for lead, particularly in acidic conditions such as those created by hydrochloric acid. They react with lead ions to form low-solubility lead phosphate minerals, such as chloropyromorphite (Pb5(PO4)3Cl) [74], which immobilizes lead and reduces its bioavailability and leachability in contaminated environments [75]. In drinking water systems, phosphate dosing is employed to mitigate lead release from pipes by promoting the formation of protective lead phosphate coatings [76]. However, the effectiveness of phosphates can be influenced by various factors, including water chemistry and the presence of competing ions, which may inhibit the formation of stable lead phosphate structures [77]. Thus, while phosphates are a promising approach for controlling lead levels, careful consideration of these factors is essential for optimizing their effectiveness. Nevertheless, phosphates seems to be more effective than silicates: for example, sodium silicate acts also as a corrosion inhibitor by forming protective layers on lead surfaces, which can limit the release of lead into water systems. However, silicates performance at lower pH values (below 4) are less pronounced than at higher pH levels [78]. Computational approaches, including density functional theory (DFT) calculations, are increasingly utilized to elucidate molecular-level inhibition mechanisms and predict inhibitor performance [79,80]. Despite significant progress, challenges remain in engineering inhibitors capable of maintaining high efficiency under diverse environmental conditions over extended durations. Future research may prioritize the development of “smart” inhibitors that adapt to changing corrosive environments and leverage nanotechnology to enhance protective capabilities [67].
In summary, the application of organic compounds and amino acids as corrosion inhibitors for lead in HCl environments has demonstrated considerable potential for industrial and environmental applications. Continued innovation in this field is essential to optimize inhibitor performance and ensure alignment with environmental sustainability goals.
 |
Fig. 2 Schematic representation of inhibitors for lead corrosion in HCl environment. |
5 Modeling of lead corrosion in HCl
The corrosion of lead in hydrochloric acid at ambient temperature has been modeled using various chemical and mathematical approaches. This involves both mechanistic modeling, as demonstrated by Barradas, and mathematical modeling techniques that aim to quantify the corrosion process.
Mechanistic models focus on the underlying electrochemical reactions and physical processes that govern corrosion, while mathematical models provide a framework for simulating these processes and predicting corrosion rates under various conditions. Graedel’s work on chemical mechanisms for the atmospheric corrosion of lead enriches this understanding by detailing how environmental factors such as humidity and pollutants influence the corrosion process [20]. The model details how these elements contribute to the formation of various corrosion products, including lead oxides, carbonates, and sulfates, highlighting the dynamic nature of lead surfaces in response to atmospheric conditions, particularly in urban environments with higher levels of industrial pollutants. By incorporating the kinetics of chemical reactions and the transport of reactive species, Graedel’s approach provides a comprehensive framework for predicting the rate and extent of lead corrosion in different atmospheric settings.
Barradas employs a mechanistic modeling approach to analyze the electrochemical dissolution of lead and the subsequent formation of lead chloride on lead electrodes. The model representes the dissolution reaction by taking into account the variety of complexes 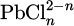 depending on the value of n. The hypothesis os steady state conditions (which means a constant value of reactivation cirrent) is made. It materiamised by an equality between dissolution rate and flux of material away from th electrode. With this model, a lead ion concentration is obtained similar to the solubility value [51]. To simulate the anodic dissolution mechanisms of lead in hydrochloric acid, Barrads utilizes electrochemical techniques alongside mathematical modeling to analyze dissolution kinetics and formation of corrosion products, particularly lead chloride (PbCl2). Barradas develops a mechanistic model that accounts for electrochemical reactions occurring at the electrode interface, emphasizing the role of ion transport and concentration gradients in the electrolyte. The model incorporates parameters such as current density and potential, allowing for a detailed examination of how these factors influence the rate of lead dissolution and characteristics of the PbCl2 layer that forms during the process. By comparing experimental data with model predictions, Barradas demonstrates that formation of a compact PbCl2 layer near the electrode surface significantly affects dissolution kinetics, highlighting interplay between electrochemical reactions and mass transport phenomena. This modeling effort not only provides insights into anodic behavior of lead amalgam electrodes but also lays groundwork for future studies aimed at mitigating corrosion in acidic environments [48]. More recently, research has focused on the corrosion of lead-tin alloys in humid environments polluted by HCl. The experimental approach has allowed for the establishment of the different stages of the corrosion process, which are quite similar to what occurs in atmospheric corrosion. The model is based on thermodynamic considerations and shows the stong role of CO2 in the lead corrosion in HCl [23,81]. Experimental data indicate that, although the model outlines the general corrosion process, it assumes the formation of compact and homogeneous layers, which does not align with reality, as cerussite tends to grow in the form of is lands. In this context, Barradas focused on the growth and morphology of the formed layers [64]. They develop a model connecting the shape of nuclei to current during the electrochemical dissolution of lead electrodes. This model is based on the premise that the nucleation process, leading to the formation of corrosion products, is influenced by the applied current density. Specifically, higher currents promote the growth of more irregular and larger nuclei, while lower currents yield smaller, more uniform structures. In addition to this, Barradas et al.’ study highlights the impact of temperature on the kinetics of lead dissolution and the formation of PbCl2 layers. The research integrates temperature effects into the mechanistic model, showing that increased temperatures accelerate the nucleation and growth processes of PbCl2, transitioning from two-dimensional to three-dimensional growth under diffusion control. By analyzing experimental data alongside this theoretical framework, Barradas et al. demonstrate a clear correlation between current density, temperature, and nucleus shape, providing insights into how these factors influence overall corrosion rates and product formation. This comprehensive approach not only enhances the understanding of electrochemical behavior but also offers a predictive tool for optimizing conditions to mitigate lead corrosion in hydrochloric acid solutions [64]. The simulation results are validated against experimental data obtained through electrochemical methods and scanning electron microscopy (SEM), demonstrating that the model accurately reflects observed phenomena, including ion concentration changes and PbCl2 precipitation dynamics. However, physically accounting for the structure of the layers is not always straightforward. Considering the presence of a corrosion product layer on the lead surface introduces two moving interfaces, which are challenging to simulate. A study employs COMSOL Multiphysics to simulate the corrosion process, focusing on the formation of a PbCl2 layer. This model incorporates a porous domain with either fixed or variable porosity, revealing that PbCl2 layer becomes more compact near electrode surface due to localized creation of lead ions [82]. The simulation results align with experimental data, validating modeling approach and indicating that both diffusion and chemical reaction mechanisms control corrosion rate in HCl solutions. However, an intermediate stage with the appearance of the first PbCl2 germs has not been taken into account. Generally, the precipitation of the first crystal germ is based on the germination-growth theory, corresponds to the spontaneous formation of crystalline germs of a solid phase in a supersaturated solution. In context of lead-acid batteries, a mathematical model developed by Boovaragavan et al. addresses effects of corrosion specifically at interface between active material and grid material of positive plate. This model employs three different approaches to incorporate corrosion effects during discharge, rest, and charge processes. The electronic conductivity of positive plate is expressed as a function of cycle number while an I-R loss term accounts for increased resistance due to passive corrosion layer formation. Comparative analysis between discharge and charge profiles with and without considering corrosion demonstrates that this modeling framework can effectively study impact of corrosion on battery performance [83]. Additionally, Stewart et al. have developed a mathematical model specifically for anodic oxidation of lead which provides insights into electrochemical processes involved during oxidation in acidic environments. This model describes how factors such as current density and electrolyte composition affect oxide layer formation on lead surfaces. Findings from this model contribute to understanding how anodic oxidation influences lead’s overall electrochemical behavior and susceptibility to corrosion in environments like HCl solutions [84].
depending on the value of n. The hypothesis os steady state conditions (which means a constant value of reactivation cirrent) is made. It materiamised by an equality between dissolution rate and flux of material away from th electrode. With this model, a lead ion concentration is obtained similar to the solubility value [51]. To simulate the anodic dissolution mechanisms of lead in hydrochloric acid, Barrads utilizes electrochemical techniques alongside mathematical modeling to analyze dissolution kinetics and formation of corrosion products, particularly lead chloride (PbCl2). Barradas develops a mechanistic model that accounts for electrochemical reactions occurring at the electrode interface, emphasizing the role of ion transport and concentration gradients in the electrolyte. The model incorporates parameters such as current density and potential, allowing for a detailed examination of how these factors influence the rate of lead dissolution and characteristics of the PbCl2 layer that forms during the process. By comparing experimental data with model predictions, Barradas demonstrates that formation of a compact PbCl2 layer near the electrode surface significantly affects dissolution kinetics, highlighting interplay between electrochemical reactions and mass transport phenomena. This modeling effort not only provides insights into anodic behavior of lead amalgam electrodes but also lays groundwork for future studies aimed at mitigating corrosion in acidic environments [48]. More recently, research has focused on the corrosion of lead-tin alloys in humid environments polluted by HCl. The experimental approach has allowed for the establishment of the different stages of the corrosion process, which are quite similar to what occurs in atmospheric corrosion. The model is based on thermodynamic considerations and shows the stong role of CO2 in the lead corrosion in HCl [23,81]. Experimental data indicate that, although the model outlines the general corrosion process, it assumes the formation of compact and homogeneous layers, which does not align with reality, as cerussite tends to grow in the form of is lands. In this context, Barradas focused on the growth and morphology of the formed layers [64]. They develop a model connecting the shape of nuclei to current during the electrochemical dissolution of lead electrodes. This model is based on the premise that the nucleation process, leading to the formation of corrosion products, is influenced by the applied current density. Specifically, higher currents promote the growth of more irregular and larger nuclei, while lower currents yield smaller, more uniform structures. In addition to this, Barradas et al.’ study highlights the impact of temperature on the kinetics of lead dissolution and the formation of PbCl2 layers. The research integrates temperature effects into the mechanistic model, showing that increased temperatures accelerate the nucleation and growth processes of PbCl2, transitioning from two-dimensional to three-dimensional growth under diffusion control. By analyzing experimental data alongside this theoretical framework, Barradas et al. demonstrate a clear correlation between current density, temperature, and nucleus shape, providing insights into how these factors influence overall corrosion rates and product formation. This comprehensive approach not only enhances the understanding of electrochemical behavior but also offers a predictive tool for optimizing conditions to mitigate lead corrosion in hydrochloric acid solutions [64]. The simulation results are validated against experimental data obtained through electrochemical methods and scanning electron microscopy (SEM), demonstrating that the model accurately reflects observed phenomena, including ion concentration changes and PbCl2 precipitation dynamics. However, physically accounting for the structure of the layers is not always straightforward. Considering the presence of a corrosion product layer on the lead surface introduces two moving interfaces, which are challenging to simulate. A study employs COMSOL Multiphysics to simulate the corrosion process, focusing on the formation of a PbCl2 layer. This model incorporates a porous domain with either fixed or variable porosity, revealing that PbCl2 layer becomes more compact near electrode surface due to localized creation of lead ions [82]. The simulation results align with experimental data, validating modeling approach and indicating that both diffusion and chemical reaction mechanisms control corrosion rate in HCl solutions. However, an intermediate stage with the appearance of the first PbCl2 germs has not been taken into account. Generally, the precipitation of the first crystal germ is based on the germination-growth theory, corresponds to the spontaneous formation of crystalline germs of a solid phase in a supersaturated solution. In context of lead-acid batteries, a mathematical model developed by Boovaragavan et al. addresses effects of corrosion specifically at interface between active material and grid material of positive plate. This model employs three different approaches to incorporate corrosion effects during discharge, rest, and charge processes. The electronic conductivity of positive plate is expressed as a function of cycle number while an I-R loss term accounts for increased resistance due to passive corrosion layer formation. Comparative analysis between discharge and charge profiles with and without considering corrosion demonstrates that this modeling framework can effectively study impact of corrosion on battery performance [83]. Additionally, Stewart et al. have developed a mathematical model specifically for anodic oxidation of lead which provides insights into electrochemical processes involved during oxidation in acidic environments. This model describes how factors such as current density and electrolyte composition affect oxide layer formation on lead surfaces. Findings from this model contribute to understanding how anodic oxidation influences lead’s overall electrochemical behavior and susceptibility to corrosion in environments like HCl solutions [84].
6 Conclusion
In summary, the corrosion of pure lead in hydrochloric acid presents significant challenges and implications for various industrial applications and environmental safety. The study reveals that lead exhibits complex corrosion behavior influenced by factors such as HCl concentration, temperature, and the presence of polluants. Understanding these mechanisms is crucial for developing effective corrosion protection strategies. Future research should focus on enhancing the stability of passivating layers and exploring innovative inhibitors to mitigate lead corrosion in acidic environments. This knowledge is essential not only for material preservation but also for addressing the environmental impacts associated with lead usage.
Electrochemical reactions of lead in HCl.
Funding
This study did not received any specific funding.
Conflicts of interest
The author declares no conflict of interest in regards to this article.
Data availability statement
No new data were generated.
References
- J.L. Caillerie, F. Wilmotte, Plomb et alliages de plomb, Tech. Ing. M510, M510–M511 (1993) [Google Scholar]
- J.S. Casas, J. Sordo, Lead: chemistry, analytical aspects, environmental impact and health effects, Elsevier, 2011 [Google Scholar]
- I.A. Bergdahl, S. Skerfving, Chapter 19 − Lead, in: G.F. Nordberg, M. Costa (Eds.), Handbook on the toxicology of metals (Fifth Edition), Academic Press 2022, pp. 427–493 [Google Scholar]
- P. Gottesfeld, Lead industry influence in the 21st Century: an old playbook for a “modern metal”, Am. J. Public Health. 112, S723–S729 (2022) [Google Scholar]
- Statista, Distribution of lead consumption worldwide in 2022, by end-use. https://www.statista.com/statistics/891778/distribution-of-global-lead-consumption-by-end-use/ [Google Scholar]
- Statista, Global lead industry − statistics & facts. https://www.statista.com/topics/5177/lead [Google Scholar]
- Y.L. Yu, W.Y. Yang, A. Hara et al., Public and occupational health risks related to lead exposure updated according to present-day blood lead levels, Hypertens. Res. 46, 395–407 (2023) [Google Scholar]
- P. Bača, P. Van`ysek, Issues concerning manufacture and recycling of lead, Energies 16, 4468 (2023) [Google Scholar]
- C. Lageot, C. Feugeas, Etude de la corrosion des sceaux en plomb, ArchéoSci. 1, 151–153 (1981) [Google Scholar]
- J. Verney, Problèmes de Corrosion du Plomb en Présence d’eau, sous differents types de composition et à différents températures, Trib. Cebedeau 25, 507 (1972) [Google Scholar]
- S.A. Bradford, S.A. Bradford, Materials selection, corrosion control, Springer, 1993, pp. 188–213 [Google Scholar]
- J. Tétreault, E. Cano, M. van Bommel et al., Corrosion of copper and lead by formaldehyde, formic and acetic acid vapours, Stud. Conserv. 48, 237–250 (2003) [Google Scholar]
- S.J. Alhassan, Corrosion of lead and lead alloys, in: Corrosion: Materials, ASM International, 2005 [Google Scholar]
- G.O. Hiers, Lead as a material for chemical equipment, Ind. Eng. Chem. 15, 467–469 (1923) [Google Scholar]
- A.J. Davidson, S.P. Binks, J. Gediga, Lead industry life cycle studies: environmental impact and life cycle assessment of lead battery and architectural sheet production, Int. J. Life Cycle Assess. 21, 1624–1636 (2016) [Google Scholar]
- B. Schotte, A. Adriaens, Treatments of corroded lead artefacts an overview, Stud. Conserv. 51, 297–304 (2006) [Google Scholar]
- The Business Research Company, Lead Global Market Report, 2025. https://www.thebusinessresearchcompany.com/report/lead-global-market-report [Google Scholar]
- J. Švadlena, T. Prošek, K.C. Strachotová et al., Chemical removal of lead corrosion products, Mater. 13, 5672 (2020) [Google Scholar]
- R.H. Gaines, The corrosion of lead, Ind. Eng. Chem. 5, 766–768 (1913) [Google Scholar]
- T.E. Graedel, Chemical mechanisms for the atmospheric corrosion of lead, J. Electrochem. Soc. 141, 922 (1994) [Google Scholar]
- Appendix G: The atmospheric corrosion chemistry of lead, in: Atmospheric Corrosion, John Wiley & Sons, Ltd, 2016, pp. 316–326 [Google Scholar]
- M.R. Schock, Understanding corrosion control strategies for lead, J. − Am. Water Works Assoc. 81, 88–100 (1989) [Google Scholar]
- F. Lequien, G. Moine, A. Lequien et al., What happens when a Pb-Sn coating deposited on low carbon steel is exposed in an HCl-polluted wet environment? Development of a corrosion mechanism, Mater. Corros. 73, 1459–1473 (2022) [Google Scholar]
- A. Niklasson, L.G. Johansson, J.E. Svensson, Influence of acetic acid vapor on the atmospheric corrosion of lead, J. Electrochem. Soc. 152, B519 (2005) [Google Scholar]
- A. Niklasson, L.G. Johansson, J.E. Svensson, Atmospheric corrosion of lead: the influence of formic acid and acetic acid vapors, J. Electrochem. Soc. 154, C618 (2007) [Google Scholar]
- ISO, Peintures et vernis − Anticorrosion des structures en acier par systèmes de peinture − Partie 2 : Classification des environnements. ISO 12944-2:2017 [Google Scholar]
- M. Kouřil, T. Boháčková, K.C. Strachotová et al., Lead corrosion and corrosivity classification in archives, museums, and churches, Mater. 15, 639 (2022) [Google Scholar]
- K. Kreislova, P. Fialová, T. Bohackova et al., Indoor corrosivity classification based on lead coupons, KOM-Corros. Mater. Prot. J. 65, 7–12 (2021) [Google Scholar]
- C.D. Evans, D.T. Monteith, D. Fowler et al., Hydrochloric acid: an overlooked driver of environmental change, Environ. Sci. Technol. 45, 1887–1894 (2011) [Google Scholar]
- T.A. Crisp, B.M. Lerner, E.J. Williams et al., Observations of gas phase hydrochloric acid in the polluted marine boundary layer, J. Geophys. Res. Atmos. 119, 6897–6915 (2014) [Google Scholar]
- Y.H. Kiang, Pre dict ing dew points of acid gases, Chem. Eng. 88, 127 (1981) [Google Scholar]
- J.J. Fritz, C.R. Fuget, Vapor pressure of aqueous hydrogen chloride solutions, O° to 50 °C, Ind. Eng. Chem. Chem. Eng. Data Ser. 1, 10–12 (1956) [Google Scholar]
- F.C. Zeisberg, Partial vapor pressures of aqueous HCl solutions, Chem. Metall. Eng. 32, 326–327 (1925) [Google Scholar]
- J.S. Park, S.H. Moon, Use of cascade reduction potential for selective precipitation of Au, Cu, and in hydrochloric acid solution, Korean J. Chem. Eng. 19, 797–802 (2002) [Google Scholar]
- Chm Ulaval (n.d.) Solubility Products Constants. Retrieved from http://www2.chm.ulaval.ca/gecha/chm1903/6_solubilite_solides/solubility_products.pdf (Accessed: 2025-01-16) [Google Scholar]
- A. Towarek, A. Mistewicz, E. Pilecka-Pietrusińska et al., Corrosion degradation of archaeological lead: A review and case study, J. Archaeol. Sci. Rep. 45, 103611 (2022) [Google Scholar]
- R. Salghi, M. Mihit, B. Hammouti et al., Electrochemical behaviour of lead in hydrochloric acid solution in the presence of inorganic ions, J. Iranian Chem. Res. (2009) [Google Scholar]
- I. De Ryck, E. Van Biezen, K. Leyssens et al., Study of tin corrosion: the influence of alloying elements, J. Cult. Heritage 5, 189–195 (2004) [Google Scholar]
- M. Pourbaix, Atlas of electrochemical equilibria in aqueous solutions, NACE, 1966 [Google Scholar]
- P. Delahay, M. Pourbaix, P. Van Rysselberghe, Potential-pH diagram of lead and its applications to the study of lead corrosion and to the lead storage battery, J. Electrochem. Soc. 98, 57 (1951) [Google Scholar]
- Rabald, Atlas d’Equilibres Electrochimiques, par M. Pourbaix, Directeur du Centre Belge d’Etude de la Corrosion “CEBELCOR”, Chargé de cours à l’Université Libre de Bruxelles en E. Deltbombe, J. Schmets, C. Vanleugenhaghe, Chercheurs au “Cebelcor” et Mme M. Moussard, MM. J. Besson, J.−P. Brenet, WG. Burgers, G. Charlot, R.−M. Garrels, T.−P. Hoar, F. Jolas, W. Kunz, M. Maraghini, R. Piontelli, K. Schwabe, G. Valensi, P. Van Rysselberghe, Members du “CITCE” et AL Pitaman, Officie of Naval Research “ONR” Format 4°(21 × 27) 644 Seiten, Preis kart. frs. 140,-(,-). 1963, Paris, Verlag Gauthier-Villars (1963) [Google Scholar]
- S.J. Alhassan, Corrosion of lead and lead alloys, in: Corrosion: Materials, ASM International, 2005 [Google Scholar]
- K.A. AL-Saadie, S.A. Al-Safi, D.E. Al-Mammar, Effect of (1, 4) phenylenediamine on the corrosion of Lead in 1M Hydrochloric acid solution, J. Um-Salama Sci. Women Univ. Baghdad, acceptedon, 30 (2007) [Google Scholar]
- K. Al-Saadie, The effect of linear alkyl benzene sulfonate on corrosion of aluminum, zinc and lead in 1M HCl, Iraqi Natl. J. Chem. 8, 76–86 (2008) [Google Scholar]
- A. Azizi, S.M.S. Ghasemi, A comparative analysis of the dissolution kinetics of lead from low grade oxide ores in HCl, H2SO4, HNO3 and citric acid solutions, Metall. Res. Technol. 114, 406 (2017) [Google Scholar]
- S.A. El Wanees, E.E.A. Aal, N-Phenylcinnamimide and some of its derivatives as inhibitors for corrosion of lead in HCl solutions, Corros. Sci. 52, 338–344 (2010) [Google Scholar]
- B.S. Evans, A rapid method of dissolving lead alloys preparatory to the determination of tin and antimony, Analyst 57, 554–559 (1932) [Google Scholar]
- R.G. Barradas, S. Fletcher, J.D. Porter, The anodic behaviour of lead amalgam electrodes in HCl solution, J. Electroanal. Chem. Interfacial Electrochem. 80, 295–304 (1977) [Google Scholar]
- R.G. Barradas, K. Belinko, E. Ghibaudi, Rotating ring-disc electrode studies of lead in HCl and NaCl solutions, Can. J. Chem. 53, 407–413 (1975) [Google Scholar]
- R.G. Barradas, K. Belinko, J. Ambrose, Electrochemical behavior of the lead electrode in HCl and NaCl aqueous electrolytes, Can. J. Chem. 53, 389–406 (1975) [Google Scholar]
- R.G. Barradas, K. Belinko, W. Shoesmith, Study of surface effects in the formation of lead chloride on lead electrodes in aqueous HCl by electrochemical methods and scanning electron microscopy, Electrochim. Acta 21, 357–365 (1976) [Google Scholar]
- R.G. Barradas, K. Belinko, E. Ghibaudi, Effect of dissolved gases on the Pb/PbCl2 electrode in aqueous chloride electrolytes, Chem. Phys. Aqueous Gas Sol. 357 (1975) [Google Scholar]
- J. Ambrose, R.G. Barradas, K. Belinko et al., Reactions at the lead electrode/hydrochloric acid interface, J. Colloid Interface Sci. 47, 441–454 (1974) [Google Scholar]
- A.M. Abd El-Halim, M.H. Fawzy, A. Saty, Cyclic voltammetric behaviour and some surface characteristics of the lead electrode in aqueous HCl solutions, J. Chem. Technol. Biotechnol. 58, 165–175 (1993) [Google Scholar]
- S.S. Abd El Rehim, A.M. El-Halim, E.E. Foad, Potentiodynamic and cyclic voltammetric behaviour of the lead electrode in HCl solutions, Surf. Technol. 18, 313–325 (1983) [Google Scholar]
- E.E. Abd El Aal, S. Abd El Wanees, Kinetics of anodic behaviour of Pb in HCl solutions, Corros. Sci. 51, 458–462 (2009) [Google Scholar]
- N.A. Lange, Lange’s handbook of chemistry (17th ed.) McGraw-Hill Education, 2017 [Google Scholar]
- G. Petiau, Second generation of lead-lead chloride electrodes for geophysical applications, Pure Appl. Geophys. 157, 357–382 (2000) [CrossRef] [Google Scholar]
- A.J. Bard, R. Parsons, J. Jordan, Standard potentials in aqueous solutions, Marcel Dekker, New York, 1985 [Google Scholar]
- C.G. Zoski, Handbook of electrochemistry, Elsevier, 2006 [Google Scholar]
- H.M. Abd El-Lateef, A. El-Sayed, H.S. Mohran et al., Corrosion inhibition and adsorption behavior of phytic acid on Pb and Pb-In alloy surfaces in acidic chloride solution, Int. J. Ind. Chem. 10, 31–47 (2019) [Google Scholar]
- W.A. Badawy, M.M. Hefny, S.S. El-Egamy, Effect of some organic amines as corrosion inhibitors for lead in 0.3 M HCl solution, Corrosion 46, 978–982 (1990) [Google Scholar]
- J. González, E. Laborda, Á. Molina, Voltammetric kinetic studies of electrode reactions: guidelines for detailed understanding of their fundamentals, J. Chem. Educ. 100, 697–706 (2022) [Google Scholar]
- R.G. Barradas, S. Fletcher, Temperature effects in the electrochemical behaviour of cycled lead electrodes in HCl solutions, Electrochim. Acta 22, 237–242 (1977) [Google Scholar]
- P.G. Harrison, G. Holt, Kinetic study of the reactions between lead metal and hydrogen bromide and hydrogen chloride, J. Chem. Soc. Faraday Trans. 88, 1027–1032 (1992) [Google Scholar]
- A.A. Al-Amiery, E. Yousif, W.N.R.W. Isahak et al., A review of inorganic corrosion inhibitors: types, mechanisms, and applications, Tribol. Ind. 44, 313 (2023) [Google Scholar]
- K. Bijapur, V. Molahalli, A. Shetty et al., Recent trends and progress in corrosion inhibitors and electrochemical evaluation, Appl. Sci. 13, 10107 (2023) [Google Scholar]
- M. Hamadouche, I. Boudechicha, Influence du milieu acide sur la corrosion du plomb, Faculté des Sciences et Technologies, 2020 [Google Scholar]
- L. Hamadi, S. Mansouri, K. Oulmi et al., The use of amino acids as corrosion inhibitors for metals: A review, Egypt. J. Petrol. 27, 1157–1165 (2018) [Google Scholar]
- N.H. Helal, M.M. El-Rabiee, G.M. Abd El-Hafez et al., Environmentally safe corrosion inhibition of Pb in aqueous solutions. J. Alloys Compd. 456, 372–378 (2008) [Google Scholar]
- W.A. Bodawy, M.M. Hefny, S.S. El-Egamy, Effect of some organic amines as corrosion inhibitors for lead in 0.3 M HCl solution, Corrosion (Houston, Tex.) 46, (1990) [Google Scholar]
- A.M. Abdel-Karim, A.M. El-Shamy, A review on green corrosion inhibitors for protection of archeological metal artifacts, J. Bio Tribo-Corros. 8, 35 (2022) [Google Scholar]
- E. Rocca, F. Mirambet, Des savons métalliques pour la protection du patrimoine, Actualite Chimique 312, 65 (2007) [Google Scholar]
- P. Zhang, J.A. Ryan, Transformation of Pb(II) from cerrusite to chloropyromorphite in the presence of hydroxyapatite under varying conditions of pH, Environ. Sci. Technol. 33, 625–630 (1999) [Google Scholar]
- M. Andrunik, M. Wołowiec, D. Wojnarski et al., Transformation of Pb, Cd, and Zn minerals using phosphates, Minerals 10, 342 (2020) [Google Scholar]
- J. Zhao, D.E. Giammar, J.D. Pasteris et al., Formation and aggregation of lead phosphate particles: implications for lead immobilization in water supply systems, Environ. Sci. Technol. 52, 12612–12623 (2018) [Google Scholar]
- J.D. Hopwood, H. Casey, M. Cussons et al., Spherulitic lead calcium apatite minerals in lead water pipes exposed to phosphate-dosed tap water, Environ. Sci. Technol. 57, 4796–4805 (2023) [Google Scholar]
- Headquarters Corporate (n.d.), Sodium silicate corrosion inhibitors: issues of effectiveness and mechanism [Google Scholar]
- H. Ke, C.D. Taylor, Density functional theory: an essential partner in the integrated computational materials engineering approach to corrosion, Corrosion 75, 708–726 (2019) [Google Scholar]
- I.B. Obot, D.D. Macdonald, Z.M. Gasem, Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: an overview, Corros. Sci. 99, 1–30 (2015) [Google Scholar]
- F. Lequien, G. Moine, Corrosion of a 75Sn/25Pb coating on a low carbon steel in a gaseous environment polluted with HCl: mechanism, Mater. Corros. 69, 1422–1430 (2018) [Google Scholar]
- M. Menut, F. Lequien, Modelling of lead corrosion in contact with an anaerobic HCl solution, Influence of the Corrosion Product Presence. Coatings 12, 1291 (2022) [Google Scholar]
- V. Boovaragavan, R.N. Methakar, V. Ramadesigan et al., A mathematical model of the lead-acid battery to address the effect of corrosion, J. Electrochem. Soc. 156, A854 (2009) [Google Scholar]
- L.L. Stewart, D.N. Bennion, R.M. LaFollette, Mathematical model of the anodic oxidation of lead, J. Electrochem. Soc. 141, 2416 (1994) [Google Scholar]
Cite this article as: Florence Lequien, Corrosion of pure lead in HCl at room temperature: a review, Metall. Res. Technol. 122, 510 (2025), https://doi.org/10.1051/metal/2025056
All Tables
Balance equation of the reactions involved in the corrosion mechanism of lead exposed to a humid and polluted by HCl environment.
All Figures
 |
Fig. 1 Schematic representation of lead corrosion processes in HCl environment. |
| In the text | |
 |
Fig. 2 Schematic representation of inhibitors for lead corrosion in HCl environment. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.