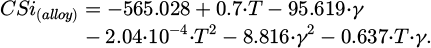| Issue |
Metall. Res. Technol.
Volume 122, Number 1, 2025
|
|
|---|---|---|
| Article Number | 115 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/metal/2024107 | |
| Published online | 06 January 2025 | |
Original Article
Thermodynamic model and electric smelting of a mixture of cake from leaching of vanadium-containing quartzites with coal beneficiation tailings
1
M. Auezov South Kazakhstan University, Kazakhstan, Shymkent, 160000 Tauke Khan 5, Kazakhstan
2
National Center on Complex Processing of Mineral Raw Materials of the Republic of Kazakhstan, Zhandosov st., 67, Almaty 050036, Kazakhstan
* e-mail: badikovasasha@gmail.com
a These authors contributed equally to this work.
Received:
13
July
2024
Accepted:
4
December
2024
The article presents the results of studies of obtaining a ferroalloy from a mixture of coal beneficiation tailings and cake from leaching of vanadium-containing quartzites (black shales) of the Balasauskandyk deposit. The study was carried out using the HSC-6.0 software package, using the Equilibrium Compositions module and the second-order rotatable planning method (Box-Hunter plan), followed by geometric optimization of equilibrium process parameters. Based on the studies on the interaction of coal beneficiation tailings and cake from leaching of vanadium-containing quartzites in the presence of iron, it was found that under equilibrium conditions, an increase in the ratio of coal beneficiation tailings to leaching cake, which allows increasing the degree of silicon extraction in FeSi, Si, is accompanied by undesirable development of SiC formation and positive decrease in the degree of gaseous SiO formation. In the temperature range of 1500–2000 °C, an increase in the tailings to the cake ratio from 0 to 1.7 increases the degree of silicon extraction into the alloy to 67.8% at 1900 °C and aluminum to 33.94% at 2000 °C. Ferrosilicon of the FeSi45 grade, with 51.1–66% Si extraction into it, is formed in the temperature range of 1703–1900 °C from a mixture of tailings and cake with their ratio from 0.38 to 1. By electric smelting of a mixture of tailings (containing 30–47% C) with cakes with a ratio from 1.0 to 0.32 together with steel cuttings, a ferroalloy − ferrosilicon of the FeSi45 grade with a content of 37–42% Si was obtained.
Key words: cake leaching / coal tailings / thermodynamic modeling / electric smelting / ferroalloy
© A.D. Badikova et al., Published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
In the modern world, environmental and resource conservation issues are of particular importance. Recycling metallurgical waste is not just a way to minimize its negative impact on the environment, but also an opportunity to expand the raw material base for various industries. The coal and metallurgical industries of Kazakhstan are the main sources of waste. In terms of coal reserves (34.2 billion tons), Kazakhstan ranks eighth in the world, and in terms of production − 11th [1–4]. As a rule, coals are beneficiated. When beneficiated, 0.3–0.4 tons of waste are formed per 1 ton of finished coal [5–8].
The composition of coal beneficiation tailings depends on the type of deposit and the type of beneficiation. For example, according to [9,10], flotation benefication tailings contain 50–67 SiO2, 10–47% C, 14–31 Al2O3, 1.0–2.0 CaO, 1.0–3.0 Fe2O3, 2.0–2.4 H2O, 0.2–0.6 S, 0.7–3 MgO, 0.3–0.7 TiO2.
Methods of processing and using coal beneficiation tailings are varied. Coal beneficiation tailings are mainly used in the construction of road foundations, embankments, filling mined-out spaces, and land reclamation [11–14]. Some coal beneficiation waste is used as an energy raw material by burning or gasification in fluidized bed furnaces, plasma furnaces, etc. For example, high-ash coal beneficiation waste in a dusty state is burned at power plants, thereby reducing fuel costs and reducing emissions of sulfur and nitrogen oxides into the environment [15–19]. Technologies were developed for using the organic component of coal beneficiation waste by pyrolysis to obtain liquid and gaseous products that are used to produce artificial liquid fuels, oils, ammonia synthesis, and lubricants [20–22].
Coal enrichment waste is successfully used for production of bricks, concrete and other building materials [23–29]. When burning of refractories, the organic components of coal enrichment tailings burn out, forming pores and reducing the density of the material, on this basis, the tailings are used as a combustible additive to raw materials in production of ceramic products: bricks, paving tiles, roofing tiles. Technologies were developed for the use of tailings in the production of artificial porous fillers (agloporite, expanded clay) and lightweight concrete based on them [30,31]. Options were proposed for using coal flotation benefication tailings as organomineral fertilizers, as well as a sorbent for wastewater treatment [32–36]. Bioleaching of coal benefication tailings with Bacillus mucilaginosus made it possible to increase the utilization rate of silicon from coal tailings to 93%, converting insoluble forms of silicon into compounds available to plants [37].
Tailings containing manganese, nickel, cobalt, vanadium, chromium, molybdenum, gold, iron and other valuable metals are an additional source of raw materials for metallurgical industry [38,39]. For example, a technology was proposed for processing waste from a coal beneficiating plant using gravity-magnetic technology, producing a gravity concentrate with an iron content of 25.3% and an extraction of 53.15%, and then using the wet magnetic separation method of iron-containing concentrate with a total iron content of 63.29% and secondary fuel-coal-containing concentrate with an ash content of 31.7% [40]. A technology for extracting alumina from coal benefication tailings containing a significant amount of aluminum oxide was proposed, which includes preparation of the charge, sintering of the waste with chalk and soda, leaching of the sinter, desiliconization of the aluminate solution, carbonization, filtering and washing of the aluminum hydroxide precipitate, and its thermal decomposition. The proposed technology showed the possibility of extracting 80–85% of the aluminum oxide contained in the material [41].
The possibility of extracting valuable metals such as manganese, nickel, cobalt, chromium, lead and zinc was proved by leaching various coal benefication tailings in a disintegrator. However, despite the high degree of extraction, the concentration of metals in the resulting solutions is quite low, which makes the process economically inexpedient [42–44].
The presence of carbon in coal beneficiation waste allows to consider the waste as potential raw materials for recovery metallurgical processes [45–48]. Moreover, the presence of volatiles in coal beneficiation tailings increases their reactivity. The increased specific resistance of tailings [49] allows to work with a lower electrode seat when smelting ferroalloys, reduces silicon losses with the gas phase.
In the metallurgical industry, waste, in addition to various sludges and slags, includes leaching cakes. Leaching cake is a by-product of hydrometallurgical processing, which is also a technogenic and valuable resource. The National Center for Complex Processing of Mineral Raw Materials of the Republic of Kazakhstan is developing a technology for hydrometallurgical processing of quartzites of the Bolshoi Karatau with the extraction of molybdenum and vanadium. The resulting cake contains up to 80% SiO2, 15–16% C and 0.08–0.1% V [50–53], allows it to be classified as a promising raw material for smelting silicon ferroalloys.
The use of waste from processing plants as raw materials for metallurgy is a promising direction for industrial development, which allows to solve the problem of waste disposal, reduce the burden on the environment and production costs, and bring a significant economic effect. In particular, the replacement of coke in the production of ferroalloys with coal benefication tailings has significant economic potential: the cost of 1 ton of carbon in metallurgical coke (85% C) is 153,000 KZT (≈300 $), while the cost of 1 ton of carbon in benefication tailings (30% C) is 8,400 KZT (≈17 $). Thus, the use of benefication tailings as a source of carbon is approximately 18 times cheaper than coke.
The aim of the study was to determine the possibility of obtaining a ferroalloy from a mixture of coal beneficiation tailings (CBT) and cake from leaching of vanadium quartzites from the Balasauskandyk deposit (LC).
2 Material and methods
The study was conducted using the HSC-6.0 software package (developed by Outokumpu Research Oy, Finland) [54], using the Equilibrium Compositions module. The equilibrium degree of distribution of substances was calculated using the algorithm developed at “Metallurgy” chair, M. Auezov South Kazakhstan University [55]. The second-order rotatable planning (Box-Hunter plans) was also used, with subsequent geometric optimization of equilibrium process parameters [56–59].
Thermodynamic modeling was carried out in the temperature range from 500 to 2000 °C with a step of 100 °C at a pressure of 1 bar.
The chemical composition of the starting materials is given in Table 1. The work used cake from autoclave sulfuric acid leaching of vanadium-containing quartzites from the Balasauskandyk deposit and tailings from dry (gravity) benefication of low-ash long-flame coal from the Shubarkol deposit.
The influence of temperature, the CBT/LC ratio (γ) on the equilibrium degree of element distribution (α, %) and their concentration in the alloy (C, %) was determined. Initially, the system (CBT):( LC) 1:1–iron was considered. The iron content in the system was 37.80% of the CBT and LC mixture mass.
The electric smelting of the leaching cake and coal beneficiation tailings mixture was carried out in a single-electrode arc furnace with a capacity of up to 15 kVA. The description and methodology of the electric smelting were described in detail in [60,61]. Before the smelting, the leaching cake and beneficiation tailings were pelletized with 3.7% bentonite clay and dried for an hour at a temperature of 120 °C. The silicon content in the alloy was determined by the pycnometric method, as well as by the atomic adsorption method using an AAS-1 device (Germany), as well as on a JSM − 6490LV scanning electron microscope with an INSAEnergy energy-dispersive microanalysis system (Japan).
Chemical composition of starting materials, %.
3 Results and discussion
Figure 1 shows the primary information on the quantitative distribution of substances in the system under consideration, obtained by the HSC-6.0 software package.
It is evident that, depending on the temperature, the main substances in the system are SiO2, C, CO(g), Fe, FeSi, Al2O3, Al2SiO5, FeSiO3, SiO(g), CaSiO3, Si, SiC, MgSiO3, S, CaS, FeO, CS2(g), Fe3Si, Al, FeSi2, FeSi2.33, COS(g), H2(g), S2(g), BaSiO3, CS(g), Mg(g), Fe5Si3, CO2(g), MgO, Al(g), H2O(g), FeSi2.43, VSi2, V, VC0.8, VC0.88, VC, VC0.9, CaO, Si(g).
Figure 2 shows information on the equilibrium distribution of silicon and aluminum, from which it is evident that silicon transforms into FeSi at a temperature of >1200 °C. The maximum of this transition (35.58%) occurs at 1900 °C. Silicon transforms into the elemental state starting at 1300 °C, increasing to 28.94% at 1900 °C. Silicon transforms into undesirable products − SiC in the range of 1500–1800 °C by 17.26% (1700 °C), and into SiO(g) starting at 1400 °C increases to 35.85% at 2000 °C.
The transition of aluminum into the elemental state increases with increasing temperature from 1500 °C to 2000 °C up to 33.94%. Aluminum transforms into the gaseous state by 4.92% at 2000 °C.
It is evident from Figure 3 that in the temperature range of 500–1000 °C vanadium transforms into carbides: VC0.8, VC0.88, VC0.9, VC. At 1300–1400 °C all vanadium is in the elemental state. Then, with an increase in the temperature to 1700 °C, VSi2, VC, VC0.8, VC0.88, VC0.9 are formed.
The main part of iron at the temperatures above 1500 °C transforms into FeSi.
The influence of temperature on the elements’ extraction degree into the alloy and the metals’ concentration is shown in Figure 4. It is evident that the silicon extraction degree into the alloy Σ FeSi, Si, Fe3Si, Fe5Si3, FeSi2.33, VSi2, V5Si3) is 67.18% at 1900 °C, and aluminum is 33.94% at 2000 °C. The silicon content in the alloy increases with increasing temperature, amounting to 45.76% at 1800 °C. The maximum concentration of aluminum in the alloy (3.94%) is observed at 2000 °C.
Figure 5 shows the areas of obtaining grade ferrosilicon [62], from which it is evident that at the temperature of 1400–1430 °C it is possible to form ferrosilicon of the FeSi10 grade, at 1430–1475 °C − ferrosilicon of the FeSi15 grade, at 1475–1550 °C − ferrosilicon of the FeSi25 grade, at 1725–1935 °C − ferrosilicon of the FeSi45 grade (with Al content of <2%). The extraction of Si into the alloy in this temperature range is 66.16–67.0%. At the temperature of 1935–2000 °C it is possible to form a Fe-Si-Al ligature containing 43.23% silicon and 3.94% aluminum.
To determine the optimal conditions for the ferroalloy formation from the mixture of CBT and LC, studies were conducted with γ from 0 to 2. In this case, both the effect of γ on the extraction of Si and iron silicides into the alloy, and on the negative degree of silicon transition into SiC and SiO(g), were determined.
Figure 6 shows the effect of γ and temperature on αSi into iron silicides SiC, SiO(g), and Figure 7–into Si.
From Figures 6 and 7 it is evident that an increase in γ leads to an increase in αSi(FeSi), αSi(Si), αSi(SiС), and decreases αSi(Fe3Si), αSi(Fe5Si3), αSi(SiО). The dependence of αSi(FeSi2.33) is more complex. The maximum of the transition of Si to FeSi2.33 (1.85%) is observed at 1900 °C. The main substances determining the behavior of silicon in the system at high temperatures are FeSi, SiC, SiO and Si. To reduce the phenomenon of SiC formation, the process should be carried out at 1800–1900 °C with γ = 0–1 or at a higher temperature with γ>1.0 (up to 2.0), and to reduce silicon losses with SiO, the process should be carried out with γ = 1.7–2.0 at 1700–1800 °C. Based on the noted different nature of the influence of γ on the silicon extraction, the main characteristic of the process is the silicon extraction degree into the alloy in the form of ΣSi, iron and vanadium silicides (αSi(alloy)). Figure 8 shows the influence of γ and temperature on αSi(alloy).
It is evident that at the temperature above 1800 °C, an increase in γ (especially from 0 to 1.7) allows increasing αSi(alloy) (for example, at 1900 °C from 27.3 to 75.4%). γ has a positive effect on αAl(alloy), increasing this indicator to 46.6–50.1% in the temperature range of 1900–2000 °C (Fig. 8B).
Figure 9 shows the change in the concentration of silicon and aluminum depending on the temperature and γ. It is evident that at a constant value of γ, the dependence of CSi(alloy) has an extreme character, which is associated with an increase in the degree of silicon loss with gaseous SiO with an increase in temperature. The maximum concentration of silicon in the alloy increases from 38.8 to 47.6% with an increase in γ from 0 to 2. This dependence is described by the equation:
At 2000 °C, increasing γ from 0 to 2 increases СAl(alloy) from 0.1 to 7.15%.
In order to determine the optimal equilibrium parameters of the influence of temperature and the ratio of raw components on αSi(alloy) and CSi(alloy), further studies were conducted using the second-order rotatable planning method. The influence of temperature (T, °C) and the ratio of components (γ) on αSi(alloy) and CSi(alloy) were carried out in the intervals given in Table 2, the planning matrix and the results of the studies are in Table 3.
Based on the data in Table 3, the following adequate second-order regression equations were obtained:
The adequacy of the obtained equations is evidenced by the value of the Fisher criterion, the tabular value of which with an error of ≤5% is less than 6.59. According to equations (1) and (2), the Fisher criteria are 6.43 and 6.51, respectively.
Using the obtained regression equations, 3D models and horizontal sections of the response surface were constructed using the MathCAD program (Fig. 10).
It is evident from the figures that the silicon extraction degree into the alloy at 1900 °C is 66.6%. Using horizontal sections of the response surface, graphical optimization was performed and the formation areas of the FeSi45 grade ferrosilicon were found. The FeSi45 grade ferrosilicon containing 41–47% silicon according to State standard 1415–93 is formed in the abcd area of Figure 11. Figure 11 shows a combined picture of the influence of temperature and γ on αSi(alloy) and CSi(alloy) with the formation of the FeSi45 grade ferrosilicon. Table 4 shows the technological values of the selected areas.
Ferrosilicon of the FeSi45 grade (41.0–44.5% Si) is formed in the temperature range of 1703–1900 °C, at γ 0.38–1, while the silicon extraction degree is 51.1–60.0%. Ferrosilicon of the FeSi45 grade with an increased silicon content from 43.7 to 46.2% can be obtained at 1760–1900 °C and γ −0.73–1.
By the electric smelting (Fig. 12) of the leaching cake and coal beneficiation tailings mixture with γ = 1 in the presence of steel cuttings (97.6% Fe, 0.48% Si, 0.4% Mn, 1.5% C), an alloy with a content of 39–42% Si, corresponding to ferrosilicon of the FeSi45 grade, was obtained.
The second smelting was carried out with a mixture of cakes and coal gravity beneficiation tailings, which contained 48.7% C, 6.92% volatiles, 42.6% ash (39.8% SiO2, 0.4% CaO, 1.4% Fe2O3, 0.8% Al2O3, 0.78% H2O, 1% S, 0.2% others). The ratio (γ) was 0.32 and the amount of steel cuttings was 50% of the mass of cake and coal beneficiation tailings. Figure 13 shows a crucible fracture with ferroalloy and crushed ferroalloy. The smelted ferroalloy contains 37.8–44.4% silicon, which corresponds to ferrosilicon of the FeSi45 grade. The silicon extraction degree in the ferroalloy was 74.8%.
 |
Fig. 1 The influence of temperature on the quantitative distribution of silicon in the (γ = 1)-Fe system. |
 |
Fig. 2 The influence of temperature on the equilibrium distribution of silicon (A) and aluminum (B) in the (γ = 1)-Fe system. |
 |
Fig. 3 The influence of temperature on the equilibrium distribution of vanadium (A) and iron (B) in the (γ = 1)-Fe system. |
 |
Fig. 4 The influence of temperature on the degree of extraction (A) and concentration (B) of elements in the alloy in the (γ = 1)-Fe system. |
 |
Fig. 5 Different ferrosilicon grades’ formation areas. |
 |
Fig. 6 The influence of γ and temperature on the silicon extraction degree in FeSi (A), Fe3Si(B), Fe5Si3(C), Fe2.33(D), SiC (E) and SiO(g) (F). |
 |
Fig. 7 The influence of γ and temperature on the silicon transition to the elemental state. |
 |
Fig. 8 The influence of γ and temperature on the extraction of silicon (A) and aluminum (B) into the alloy. |
 |
Fig. 9 The influence of γ and temperature on the concentration of silicon (A) and aluminum (B) in the alloy. |
Levels of factors and ranges of their variation in the study of the influence of temperature and the ratio of components on the production of ferroalloy.
Matrix for planning experiments and their results.
 |
Fig. 10 The influence of γ and temperature on αSi(alloy) (А) and С Si(alloy) (В). |
 |
Fig. 11 Combined information on the influence of γ and temperature on the silicon extraction degree into the alloy and on the concentration of silicon in it. |
 |
Fig. 12 Electric smelting of the mixture of quartzite leaching cake and coal beneficiation tailings. |
 |
Fig. 13 Photograph of the crucible fracture (A) and crushed ferroalloy (B). |
4 Conclusion
Based on the conducted studies on the interaction of coal beneficiation tailings and cake from leaching of vanadium-containing quartzites in the presence of iron, the following conclusions can be made:
-
Under equilibrium conditions:
Depending on the temperature and composition of the mixture, the products are FeSi, SiO2, C, CO(g), Fe, Al2O3, Al2SiO5, FeSiO3, SiO(g), CaSiO3, Si, SiC, MgSiO3, S, CaS, FeO, CS2(g), Fe3Si, Al, FeSi2, FeSi2.33, COS(g), H2(g), S2(g), BaSiO3, CS(g), Mg(g), Fe5Si3, CO2(g), MgO, Al(g), H2O(g), FeSi2.43, VSi2, V, VC0.8, VC0.88, VC, VC0.9, CaO, Si(g);
An increase in the ratio of coal beneficiation tailings to leaching cake, which allows increasing the silicon extraction degree in FeSi, Si, is accompanied by undesirable development of SiC formation and a positive decrease in the degree of formation of gaseous SiO;
In the temperature range of 1500–2000 °C, an increase in the ratio of tailings to cake from 0 to 1.7 increases the silicon extraction degree into the alloy to 67.8% at 1900 °C and aluminum to 33.94% at 2000 °C;
Ferrosilicon of the FeSi45 grade, with the extraction of 51.1–66% silicon, is formed in the temperature range of 1703–1900 °C from the mixture of tailings and cake with their ratio from 0.38 to 1.
During the electric smelting of the beneficiation tailings with cake (with the ratio from 1.0 to 0.32) in the presence of steel cuttings, the ferroalloy − ferrosilicon of the FeSi45 grade with the content of 37–42% Si is obtained.
The presented study is the initial stage of a comprehensive work aimed at creating an innovative technology for processing vanadium quartzite leaching cake. The results obtained allow to conclude that further research is promising. The next stage in the development of the technology will be experimental work on the use of other types of carbon-containing raw materials, determination of optimal process parameters and large-scale laboratory tests.
Funding
This study is conducted within the framework of the project AP23489340 of the Ministry of Science and Higher Education of the Republic of Kazakhstan.
Conflicts of interest
The authors have nothing to disclose.
Data availability statement
This article has no associated data generated and/or analyzed.
Author contribution statement
Conceptualization, A. D. Badikova and V. M. Shevko; Methodology, V. M. Shevko; Software, V. M. Shevko; Validation, A. D. Badikova and V. M. Shevko; Formal Analysis, A. D. Badikova and V. M. Shevko; Investigation, A. D. Badikova; Resources, V. M. Shevko and D. K. Aitkulov; Data Curation, V. M. Shevko; Writing − Original Draft Preparation, A. D. Badikova; Writing − Review & Editing, A. D. Badikova, M. Shevko and D. K. Aitkulov; Visualization, A. D. Badikova; Supervision, V. M. Shevko and D. K. Aitkulov; Project Administration, A. D. Badikova; Funding Acquisition, D. K. Aitkulov.
References
- A. Akhunbayev, Sostoyaniye i perspektivy ugol’noy promyshlennosti Kazakhstana [State and prospects of the coal industry of Kazakhstan], Min. Metall. Indust. 8, 26–29 (2017) (in Russ.) [Google Scholar]
- Z. Atakhanova, S. Azhibay, Assessing economic sustainability of mining in Kazakhstan, Miner. Econ. 36, 719–731 (2023) [Google Scholar]
- R.A. Alshanov, Kazakhstan na mirovom mineral’no-syr’yevom rynke: problemy i ikh resheniye : analiz i prognoz [Kazakhstan in the global mineral resources market: problems and their solutions: analysis and forecast], Prínt-S, Almaty, 2004, (in Russ.) [Google Scholar]
- B. Akhmetzhanov, K.B. Tazhibekova, A.A. Shametova, Ugol’naya promyshlennost’ Kazakhstana: problemy i perspektivy [Coal industry of Kazakhstan: problems and prospects], Bull. Karaganda Univ. Ser. Econ. 4, 63–69 (2018) [Google Scholar]
- T.I. Chernyshova, N.V. Alpatov, Polucheniye toplivnykh briketov iz otkhodov metallurgicheskogo proizvodstva [Production of fuel briquettes from metallurgical waste], Aktual’nyye voprosy sovremennoy nauki [Current issues of modern science] 29, 174–183 (2013) (in Russ.) [Google Scholar]
- M.Y. Shpirt, V.B. Artem’yev, S.A. Silyutin, Ispol’zovaniye tverdykh otkhodov dobychi i pererabotki ugley [Use of solid waste from coal mining and processing], Gornoye delo, Moscow, 2013, (in Russ.) [Google Scholar]
- M.Y. Shpirt, E.G. Gorlov, A.V. Shumovskiy, Concept of a technological complex for coal waste processing with the production of a wide range of commercial products, Solid Fuel Chem. 53, 352–356 (2019) [CrossRef] [Google Scholar]
- G.B. Skripchenko, R.Y. Kleyman, M.Y. Shpirt et al., Phase composition of coal mining and preparation wastes and its role in determining the trends of their usage, Coal Sci. Technol. 24, 1637–1639 (1995) [CrossRef] [Google Scholar]
- N.Y. Svechnikova, S.V. Yudina, N.I. Mamedalina, Analiz otkhodov flotatsionogo obogashcheniya uglya [Analysis of coal flotation enrichment waste], Theory Technol. Metall. Prod. 1, 19–21 (2015) [Google Scholar]
- R. Ya. Kleiman, G.B. Skripchenko, M. Ya. Shpirt et al., Quantitative phase analysis of coal-mining and enrichment wastes, Solid Fuel Chem. 23, 126–128 (1989) [Google Scholar]
- V.I. Golik, Technology of the environmentally correct recultivation of the mine surface with leaching of the coal enrichment tailings, Bezopasnost’ Truda v Promyshlennosti, 4, 13–17 (2022) [Google Scholar]
- B. Klojzy-Karczmarczyk, J. Mazurek, Proposals to extend actions to the management of waste rock from hard coal mining, Zeszyty Naukowe Instytutu Gospodarki Surowcami Mineralnymi Polskiej Akademii Nauk, 98, 151–166 (2017) [Google Scholar]
- J. Feliks, B. Klojzy-Karczmarczyk, M. Wiencek, Granulating coal sludge and their mixtures to improve transport properties, Zeszyty Naukowe Instytutu Gospodarki Surowcami Mineralnymi i Energiа Polskiej Akademii Nauk, 104, 173–188 (2018) [Google Scholar]
- A. Bolatova, A. Kuttybayev, A. Каinazarov et al., Use of mining and metallurgical waste as a backfill of worked-out spaces, News Natl. Acad. Sci. Republic Kazakhstan, Ser. Geol. Tech. Sci. 1, 33–38 (2022) [Google Scholar]
- V. Murko, V. Hamalainen, The development of environmentally friendly technologies of using coals and products of their enrichment in the form of coal water slurries, E3S Web Conf. 21, 01029 (2017) [CrossRef] [EDP Sciences] [Google Scholar]
- V. Messerle, M. Orynbasar, K. Umbetkaliev et al., Gasifi cation of carbon-contaning waste in a plasma-chemical reactor, Combustion Plasma Chem. 21, 191–200 (2023) [Google Scholar]
- F. Cheng, Y. Zhang, G. Zhang et al., Eliminating environmental impact of coal mining wastes and coal processing by-products by high temperature oxy-fuel CFB combustion for clean power Generation: a review, Fuel, 373, 132341 (2024) [CrossRef] [Google Scholar]
- V.N. Petukhov, N.Y. Svechnikova, S.V. Yudina et al., Utilization of coal-flotation wastes at OAO TsOF Belovskaya, Coke Chem. 59, 200–203 (2016) [CrossRef] [Google Scholar]
- N.V. Panishev, V.A. Bigeyev, Ye.S. Galiulina, Perspektivy utilizatsii khvostov ugleobogashcheniya i tverdykh otkhodov teplovykh elektrostantsiy [Prospects for the utilization of coal tailings and solid waste from thermal power plants], Theory Technol. Metall. Prod. 2, 69–77 (2015) [Google Scholar]
- M. Pashkevich, I. Sverchkov, M. Chukaeva, Current problems in integrated processingand-utilization of hard-to-process ores and man-induced mineral raw materials (The Plaksin’s Readings — 2017), Obogashchenie Rud, 6, 54–57 (2017) [CrossRef] [Google Scholar]
- L. Hai-bing, H. He-long, F. Xing-min et al., Characteristics and kinetics of the pyrolysis of coking coal tailings, J. Environ. Eng. Technol. 2, 525–530 (2012) [Google Scholar]
- S. Liu, X. Fu, F. Zhu et al., Catalytic pyrolysis of coking-coal tailings for production of hydrogen-rich fuel gas, Chin. J. Environ. Eng. 7, 4067–4071 (2013) [Google Scholar]
- A. Yu. Stolboushkin, A.I. Ivanov, O.A. Fomina, Use of coal-mining and processing wastes in production of bricks and fuel for their burning, Procedia Eng. 150, 1496–1502 (2016) [CrossRef] [Google Scholar]
- V. Lemeshev, I. Gubin, Yu. Savel’ev et al., Utilization of coal-mining waste in the production of building ceramic materials, Glass Ceram. 61, 308–311 (2004) [CrossRef] [Google Scholar]
- S. Yagüe, I. Sánchez, R. Vigil de la Villa et al., Coal-mining tailings as a pozzolanic material in cements industry, Minerals 8, 46 (2018) [CrossRef] [Google Scholar]
- Ye.S. Abdrakhimova, Ispol’zovaniye otkhodov ugleobogashcheniya i mezhslantsevoy gliny v proizvodstve keramicheskogo kirpicha [Use of coal enrichment waste and intershale clay in the production of ceramic brick], Coal 7, 52–55 (2021) [Google Scholar]
- N.V. Boltakova, G.R. Faseeva, R.R. Kabirov et al., Utilization of inorganic industrial wastes in producing construction ceramics, review of Russian experience for the years 2000-2015, Waste Manag. 60, 230–246 (2017) [CrossRef] [Google Scholar]
- A.A. Lavrinenko, N.Y. Svechnikova, N.S. Konovnitsyna et al., Utilization of bituminous coal flotation wastes in the manufacture of ceramic brick, Solid Fuel Chem. 52, 406–410 (2018) [CrossRef] [Google Scholar]
- I.A. Denisova, N.A. Vilbitskaya, A.I. Yatsenko et al., The use of pyrite concentrate and waste formed at the brown coals enlargement, Mater. Sci. Forum 974, 336–341 (2020) [Google Scholar]
- S.N. Abdurakhmanov, Tekhnologiya polucheniya aglosporita iz flotatsionnykh khvostov ugleobogashcheniya i mineral’nogo syr’ya [Technology of obtaining agglomerate from flotation tailings of coal enrichment and mineral raw materials], P.P. Budnikov VNIISTROM, Kraskovo, 1994, [Google Scholar]
- V.Z. Abdrakhimov, N.V. Lazareva, Ispol’zovaniye otkhodov flotatsii ugleobogashcheniya v proizvodstve keramzita sposobstvuyet ekologii i rasshiryayet granitsy zemleustroystva i kadastrov [Use of coal flotation waste in expanded clay production promotes ecology and expands the boundaries of land management and cadastres], Ekspert: teoriya i praktika, 6, 40–47 (2020) [Google Scholar]
- Ye.I. Goncharuk, N. P. Tret’yak, Gigiyenicheskoye obosnovaniye dozy vneseniya otkhodov flotatsii uglya v pochvu v kachestve udobreniya [Hygienic justification for the dose of applying coal flotation waste into the soil as fertilizer, Hygiene Sanitation 10, 7–10 (1990) [Google Scholar]
- M.T. Yong, M. Babla, S. Karan, Coal tailings as a soil conditioner: evaluation of tailing properties and effect on tomato plants, Plant Growth Regul. 98, 439–450 (2022) [CrossRef] [PubMed] [Google Scholar]
- S. Soloviev, I. Semina, V. Androkhanov et al., Restoration of vegetation cover in reclaimed areas with coal preparation waste in Kuzbass, E3S Web Conf. 244, 01015 (2021) [CrossRef] [EDP Sciences] [Google Scholar]
- K. Nunes, J. Illi, V. Jurado-Davila et al., Use of coal beneficiation tailings as solid sorbents in the treatment of nitrate-contaminated real wastewater, Appl. Water Sci. 10, 93 (2020) [CrossRef] [Google Scholar]
- N.A. Korolev, I.A. Korolev, Netraditsionnyye napravleniya ispol’zovaniya otkhodov dobychi i obogashcheniya uglya [Unconventional areas of use of coal mining and beneficiation waste] Proceedings of VIII All-Russian scientific and practical conference of young scientists with international participation “Young Russia”, Kemerovo, April 19-22, 2016, Kuzbass State Technical University named after T. F. Gorbachev; Kemerovo (2016) [Google Scholar]
- Q. Zhang, L. Ma, Y. Peng et al., Sustainable bioleaching of heavy metals from coal tailings using Bacillus inaquosorum B.4: mechanistic insights and environmental implications, J. Environ. Chem. Eng. 12, 113400 (2024) [CrossRef] [Google Scholar]
- M.S. Lebzin, A.I. Bolgova, M.S. Ovsyannikov et al., Ecological aspects of deep processing of tailings of Donbass coal enrichment, News Ural State Mining Univ. 2, 96–103 (2022) [Google Scholar]
- T.G. Cherkasova, E.V. Cherkasova, A.V. Tikhomirova et al., Rare earth elements in Kuzbass coal processing wastes, Ugol, 3, 65–68 (2023) [CrossRef] [Google Scholar]
- E.S. Prokopiev, O.L. Alekseeva, Evaluating the possibility for coal-containing waste of West Siberian Iron-and-Steel Works sludge storage to be involved in processing, Earth Sci. Subsoil Use, 4, 446–457 (2022) [Google Scholar]
- N.I. Belomerya, A. Yu. Shevchenko, Ispol’zovaniye otkhodov ugleobogashcheniya dlya polucheniya glinozema [Use of coal enrichment waste to produce alumina], Proceedings of the International Scientific and Practical Conference: Environmental Problems of Industrial Megacities. Donetsk, Avdeevka, June 1-4, 2004, Donetsk, DonNTU of the Ministry of Education and Science of Ukraine, 471–475 (2004). [Google Scholar]
- V.I. Golik, Yu.I. Razorenov, Khvosty obogashcheniya uglya kak syr’ye dlya proizvodstva stroitel’nykh materialov [Coal enrichment tailings as a raw material for the production of building materials], Dry Construction Mixtures, 4, 29–32 (2013) [Google Scholar]
- M.S. Lebzin, A.I. Bolgova, M.S. Ovsyannikov et al., Ecological aspects of deep processing of tailings of donbass coal enrichment, News Ural State Mining Univ. 2, 96–103 (2022) [Google Scholar]
- V.I. Komashchenko, E.D. Vorobev, Y.I. Razorenov, Extraction of metals when recycling enrichment of ores, Bull. Tomsk Polytech. Univ. Geo Аssets Eng. 328, 18–24 (2017) [Google Scholar]
- O.T. Ibraeva, I.K. Ibraev, G.Sh. Zhaksybaeva, Directions use of waste coal flotation in metallurgical рroduction, Sci. Rev. Tech. Sci. 2, 26–31 (2016) [Google Scholar]
- B. Machulec, W. Bialik, S. Gil, Application of the mining industry wastes as raw material for melting of the complex fesial ferroalloys, Arch. Metall. Mater. 63, 975–979 (2018) [Google Scholar]
- N.Yu. Svechnikova, V.N. Petukhov, S.V. Yudina et al., Use of coal preparation waste as a reducing agent in the Romelt process, Chernye Metally, 12, 14–19 (2023) [CrossRef] [Google Scholar]
- V. Shevko, D. Aitkulov, A. Badikova, Comprehensive processing of vanadium-containing black shale tailings, Period. Polytech. Chem. Eng. 66, 617–628 (2022) [CrossRef] [Google Scholar]
- A.G. Kaliakparov, Razrabotka sposobov al’ternativnogo proizvodstva i ispol’zovaniya uglerodistykh vosstanoviteley v usloviyakh Kazakhstana [Development of methods for alternative production and use of carbonaceous reducing agents in the conditions of Kazakhstan], Zh. Abishev Chemical and Metallurgical Institute, Karaganda, 2010, [Google Scholar]
- N.M. Komekova, V.A. Kozlov, K.M. Smirnov et al., Autoclave leaching of vanadium from black shale, Metallurgist 60, 1186–1190 (2017) [CrossRef] [Google Scholar]
- S.K. Dzhumankulova, V.I. Zhuchkov, Z.A. Alybaev, Review of state and prospects for development of vanadium production in the Kazakhstan Republic, Metallurgist 64, 75–81 (2020) [CrossRef] [Google Scholar]
- A. Kali, L.T. Boshkayeva, S.K. Dzhumankulova et al., Sposoby pererabotki vanadiyevykh rud slozhnogo sostava [Methods for processing vanadium ores of complex composition], Proceedings of Satpaev Readings − 2022, Satbayev University, Almaty, Kazakhstan, 3, 207–211 (2022) [Google Scholar]
- YA.V. Grazhdanova, L.KH. Batrakova, V.A. Kozlov, Izvlecheniye vanadiya i urana iz kvartsitov Karatau metodom perkolyatsionnogo vyshchelachivaniya [Extraction of vanadium and uranium from Karatau quartzites by percolation leaching], Bull. Eng. Acad. 1, 73–77 (2003) [Google Scholar]
- A. Roine, HSC Chemistry Software, Metso Outotec, Pori, 2021. Available at: www.mogroup.com/hsc. Accessed: 12.02.2024 [Google Scholar]
- V.M. Shevko, G.M. Serzhanov, G.E. Karataeva et al., Calculation of the equilibrium distribution of elements in relation to the software package HSC-5.1. Computer program // Certificate of the Republic of Kazakhstan # 1501 (29.01.2019) [Google Scholar]
- G.M. Grinfeld, A.V. Moiseev, Methods for optimizing experiments in chemical technology, FSBEI HPE KnAGTU, Komsomolsk-on-Amur, 2014, [Google Scholar]
- S.L. Akhnazarova, V.V. Kafarov, Methods for optimizing experiments in chemical technology: Textbook for universities, Higher School, Moscow, 1985, [Google Scholar]
- A.M. Inkov, T. Tapalov, U.U. Umbetov et al., Optimization methods, SKSU, Shymkent, 2003, [Google Scholar]
- V.F. Ochkov, Mathcad 14 for students, engineers and designers, BHV-Petersburg, Saint Petersburg, 2007, [Google Scholar]
- V. Shevko, N. Mirkayev, B. Lavrov et al., Obtaining a silicon alloy from a sedimentary rock − Tripoli, J. Chem. Technol. Metall. 58, 367–375 (2023) [CrossRef] [Google Scholar]
- V.M. Shevko, R.A. Uteeva, A.B. Badikova et al., Production of ferroalloys, calcium carbide, and phosphorus from high-silicon phosphorite, Rasayan J Chem. 16, 955–963 (2023) [CrossRef] [Google Scholar]
- State standard 1415 −93, Ferrosilicon. Technical requirements and terms of delivery, Standartinform, Moscow, 2011 [Google Scholar]
Cite this article as: Alexandra D. Badikova, Viktor M. Shevko, Dosmurat K. Aitkulov, Thermodynamic model and electric smelting of a mixture of cake from leaching of vanadium-containing quartzites with coal beneficiation tailings**, Metall. Res. Technol. 122, 115 (2025)
All Tables
Levels of factors and ranges of their variation in the study of the influence of temperature and the ratio of components on the production of ferroalloy.
All Figures
 |
Fig. 1 The influence of temperature on the quantitative distribution of silicon in the (γ = 1)-Fe system. |
| In the text | |
 |
Fig. 2 The influence of temperature on the equilibrium distribution of silicon (A) and aluminum (B) in the (γ = 1)-Fe system. |
| In the text | |
 |
Fig. 3 The influence of temperature on the equilibrium distribution of vanadium (A) and iron (B) in the (γ = 1)-Fe system. |
| In the text | |
 |
Fig. 4 The influence of temperature on the degree of extraction (A) and concentration (B) of elements in the alloy in the (γ = 1)-Fe system. |
| In the text | |
 |
Fig. 5 Different ferrosilicon grades’ formation areas. |
| In the text | |
 |
Fig. 6 The influence of γ and temperature on the silicon extraction degree in FeSi (A), Fe3Si(B), Fe5Si3(C), Fe2.33(D), SiC (E) and SiO(g) (F). |
| In the text | |
 |
Fig. 7 The influence of γ and temperature on the silicon transition to the elemental state. |
| In the text | |
 |
Fig. 8 The influence of γ and temperature on the extraction of silicon (A) and aluminum (B) into the alloy. |
| In the text | |
 |
Fig. 9 The influence of γ and temperature on the concentration of silicon (A) and aluminum (B) in the alloy. |
| In the text | |
 |
Fig. 10 The influence of γ and temperature on αSi(alloy) (А) and С Si(alloy) (В). |
| In the text | |
 |
Fig. 11 Combined information on the influence of γ and temperature on the silicon extraction degree into the alloy and on the concentration of silicon in it. |
| In the text | |
 |
Fig. 12 Electric smelting of the mixture of quartzite leaching cake and coal beneficiation tailings. |
| In the text | |
 |
Fig. 13 Photograph of the crucible fracture (A) and crushed ferroalloy (B). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.