| Issue |
Metall. Res. Technol.
Volume 115, Number 4, 2018
Trends in heat treatment and surface engineering
|
|
|---|---|---|
| Article Number | 406 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/metal/2018072 | |
| Published online | 24 August 2018 | |
Regular Article
Hypothetic impact of chemical bonding on the moisture resistance of amorphous SixNyHz by plasma-enhanced chemical vapor deposition
1
CEMEF - MINES ParisTech,
1 rue Claude Daunesse,
06904
Sophia-Antipolis cedex, France
2
VISHAY,
199 Boulevard de la Madeleine,
06000
Nice, France
* e-mail: catheline.cazako@mines-paristech.fr
Received:
9
January
2018
Accepted:
6
July
2018
The relationship between the microstructure of silicon nitride and its sensitivity to moisture was studied. The effectiveness of Si-H rich and N-H rich silicon nitride layers was measured under attack from water in vapor and liquid states. For water vapor attack, samples are exposed to vapor at 85 °C with a relative humidity of 85% during 1600 hours; for liquid water attack, samples are dipped in water at 60, 85 and 100 °C for 200 hours. The water resistance of the Si-H rich and N-H rich silicon nitride layers was evaluated by measuring: (i) the thickness of the silicon dioxide formed after their oxidation with water vapor, (ii) the rate of dissolution of the silicon nitride in liquid water and (iii) the corresponding activation of energy. This evaluation was performed by coupling spectroscopic ellipsometry, infra-red and X-ray photoelectron spectrometry analyses. The results revealed that for Si-H rich layer, 10 nm of silicon dioxide was formed during the water vapor attack; for liquid water attack, a high activation energy (0.88 eV) and a low dissolution rate were observed regardless of the water temperature. For N-H rich layers, approximatively 6–8 nm of silicon dioxide was formed and a low activation energy (0.64 eV) with a high dissolution rate were observed. All of these observations lead to the conclusion that the N-H rich layers could be less resistant to moisture because the isoelectronic relationship between Si2N-H and −H2O+ facilitated their deterioration in water. Moreover, a higher rate of nanoporosity for N-H rich layers than Si-H rich layer could complete this hypothesis.
Key words: dissolution rate / silicon nitride / PECVD / moisture resistance / chemical bonding
© EDP Sciences, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
In microelectronics, moisture resistance is an important requirement. A thin film, called “passivation layer”, is used to insulate component against electromagnetic fields and to protect the moisture sensitive materials against corrosion.
Inorganic passivation layers may be made from silicon nitride (Si3N4), silicon oxide (SiO2) and silicon oxinitride (SiON). The properties of silicon nitride have been found to present a good compromise between moisture resistance and dielectric behavior [1]. The moisture-resistance of silicon nitride, however, greatly depends on its stoichiometry, hydrogen content, the nature of chemical bonding and the state of residual mechanical stress [2–4]. The characteristics of these layers are influenced by the deposition methods and parameters. Among the different procedures used to deposit silicon nitride [5], Plasma Enhanced Chemical Vapor Deposition (PECVD) is predominant. The advantage of this method is the low temperature deposition (25–400 °C) due to the plasma chemistry. The plasma is produced by a high and/or low radio frequency discharge and a gas mixture, generally composed of SiH4, NH3, N2.
The usual reaction occurring in silicon nitride PECVD is SiH4 + NH3 → SixNyHz + H2. The resulting silicon nitride is contaminated by hydrogen occurring from reagents [5,6]. Low temperature deposition is not favorable for hydrogen desorption and results in N–H and Si–H bondings, porosity and a non-stoichiometric structure. These phenomenon lead to an amorphous hydrogenated silicon nitride (SixNyHz) passivation layer [7].
Depending on the deposition conditions, it is possible to reach about 20% of hydrogen atoms in a silicon nitride PECVD layer [6–8]. In a moist environment, these hydrogen atoms improve the hydrolysis of silicon nitride and results in the formation of Si-O-Si, Si-OH and NH3 products [1,3,9]. The formation of Si-O-Si and Si-OH bonds enhances porosity and increases water diffusion in the silicon nitride passivation layer. If hydrogenated silicon nitride remains in contact with water, hydrolysis will lead to dissolution of this layer [1,4,10–12].
The evaluation of moisture resistance is frequently made by water vapor attack such as the pressure cooker test with 121 °C, 2 atm and 100% RH (Relative Humidity) [3], water vapor transmission test or standardized classical moisture test (i.e. 85 °C /85% RH). During these tests, the performance of the layer can be evaluated by electric monitoring of the moisture sensitive materials (i.e. in the case of resistors, the monitoring of the ohmic drift) [11] or by the water vapor transmission rate [13]. This evaluation is empirical and provides no information about the local interactions between water and the silicon nitride layer. Fine characterizations like measurements of relative intensity of Si-O-Si peak at 1070 cm−1 in Infra-Red (IR), and the depth profile of Si/O and Si/N atomic ratio by X-ray Photoelectron Spectroscopy (XPS) can be used to study the evolution of the chemical structure under moisture attacks [3]. The general observations are an increasing in Si-O-Si peak with the moisture exposition time and a high Si/O atomic ratio at the first nanometers of the layer [3,9,14,15]. Less usual moisture resistance evaluations were made using condensed water (or liquid water) [1,4,10–12]. Silicon nitride layers were soaked in water at different temperatures. For a 1/0.9 Si/N layer soaked in 100 °C condensed water, the dissolution rate is about 6.3 nm/h with an activation energy of 0.63 eV [4]. All of these characteristics greatly depend on the deposition conditions.
In this work, we focused on the influence/relationship between the chemical structure of silicon nitride and its sensitivity to water. An appropriate use of Spectroscopic Ellypsometer (SE) allowed to quantify the surface oxidization and the dissolution rate of silicon nitride with water (vapor and liquid state). Complementary techniques like IR and XPS were employed to characterize the layer before and after moisture tests. Furthermore, with these techniques, we tried to identify the weak point of the microstructure leading to moisture sensitivity and we finally quantified the robustness of each layer.
The sample preparation with various chemical compositions necessitated the adjustment of the deposition conditions. For this reason, radio frequency and the mixture reagent were modified.
2 Methods
The amorphous silicon nitride films were deposited in a parallel plate reactor made by Oxford Instruments. This apparatus can supply a radio frequency of 0.10 MHz or/and 13.56 MHz. Silicon nitride deposition was made on silicon wafers. For IR analysis, we used FZ (Float-Zone process) double-side polished {100} silicon wafers whereas for XPS and SE analysis, CZ (Czochralski process) single-side polished p-type {100} silicon wafers were used.
2.1 Film deposition and characterization
The deposition conditions are outlined in Table 1. The deposition temperature is 250 °C and the thickness of as-deposited silicon nitride is about 250 nm. The thicknesses before/after the moisture test and the refractive index were measured with a SE provided by Woolam and were identified by a model consisting of SiO2/Cauchy film/silicon substrate [14]. The IR spectrum of each silicon nitride film was measured with a Brüker infrared spectrometer in transmission mode. Four vibrations were identified: Si-N (900 cm−1), Si-N-H (3350 cm−1 and 1170 cm−1) and Si-H (2150 cm−1). By using the Landford and Rand procedure [6], the hydrogen content of each film was determined from absorption peaks of Si-H (2150 cm−1) and N-H (3340 cm−1). The vibrations of Si-O bonding at 1070 cm−1 and 800 cm−1 from the native silicon dioxide cannot be detected by the infrared transmission mode because of its low relative quantity. The atomic ratio Si/N and the depth chemical composition profile of films were estimated using an XPS with a monochromatic Al Kα source. Depth profiles were obtained by sputtering the surface with a monoatomic Ar+ ion beam (500 eV and 15 μA) with a theoretical etch rate of 0.1 nm/s on Ta2O5. An ellipsoid surface of 400–800 μm2 was analyzed in the center of the 1.3*2.6 mm2 sputtered surface. After moisture tests, the residual thickness measured by SE was divided by the total etching time and resulted in the sputtering rate. These calculations allow the analyzed depth to be determined. During XPS analysis, a low energy flood-gun is used in order to reduce the charge effect. The pass energy is 50 eV for the detailed spectra. Sensitivity factors for chemical quantification of Si 2p and N 1s are 0.817 and 1.800 respectively. The residual mechanical stress of layers was determined by measuring the bending of the silicon nitride film on double sided silicon wafer substrate. The stress is calculated by curvature angle of the sample and the Stoney relation.
Two moisture tests were done:
-
water vapor attack at 85 °C /85% RH for aging times up to 1600 hours. The thickness of silicon dioxide induced by the water vapor attack on silicon nitride was measured by SE
-
liquid water attack: silicon nitride layers were immersed in water and the thickness before and after the water attack was measured by SE. The silicon nitride dissolution rate was determined by the dissolved thickness as a function of soak time (up to 200 hours) in water. This test was done at different water temperatures (60, 85 and 100 °C). With the collected data and Arrhenius Law (Eq. (1)), the activation energy of dissolution reaction was determined. The chemical composition in the depth layer was characterized by XPS.
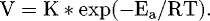 (1)
V = dissolution rate (m/h), K = kinetic constant (m/h), Ea = activation energy (J.mol−1), R = 8,314 J ∙ K−1 ∙ mol−1, T = temperature (K).
(1)
V = dissolution rate (m/h), K = kinetic constant (m/h), Ea = activation energy (J.mol−1), R = 8,314 J ∙ K−1 ∙ mol−1, T = temperature (K).
Deposition conditions.
3 Results and discussion
3.1 The radio frequency impacts the layer stoichiometry and the mechanical residual stress
Table 2 shows that the Si/N atomic ratio of layers is superior to the 0.75 atomic ratio of the stoichiometric Si3N4. It reveals that the deposited layer is silicon-rich. Furthermore, the HF sample contains more silicon than the other samples. It is tensile stressed with a high refractive index and deposition rate. By contrast, the LF and LF Free NH3 samples contain more nitrogen and hydrogen than the HF sample. The layer is compressive stressed with a low refractive index and deposition rate.
These results bring to light the influence of radiofrequency on the stoichiometry and the microstructure of silicon nitride deposited by PECVD. The composition of the layer after PECVD process depends on the plasma composition. The formation of the plasma is induced by the collision between an electron and a gaseous molecule; the resulted plasma is composed of neutral and ionic particles. During the deposition, the radiofrequency impacts the collision between particles:
-
at high frequency (> 4 MHz), electrons are in movement whereas ionic particles stay immobile; only neutral particles participate to the deposition. As the dissociation energy of SiH4 (Edissociation = 3,1 eV) is lower than N2 (Edissociation = 9,9 eV), the collision of electron gives more silicon-rich neutral particles. The deposited layer is silicon-rich. During the deposition, there is desorption of hydrogen (from Si-H and N-H) and cross-linking of Si-N bonding, which cause shrinkage of the film. The layer formed is silicon-rich and tensile stressed;
-
at low frequency (< 4 MHz) in addition to electron, ionic particles are in movement. Inelastic collision between electron and particles are produced. The deposition is composed of radical and nitrogen-rich ionic bombardment. The ionic bombardment produced a high compressive stressed layer.
As-deposited layer characteristics.
3.2 The mixture choice decreases the stress and the hydrogen content
Table 2 shows that LF Free NH3 (mixture SiH4/N2) and LF (mixture SiH4/NH3/N2) samples contain approximately similar Si and N atomic percent.
However, the sample elaborated with the mixture SiH4/N2 contains less hydrogen than LF samples elaborated with the mixture SiH4/N2/NH3. For the same radio frequency, but with a different mixture (SiH4/NH3/N2 or SiH4/N2), the absolute value of stress decreases with the hydrogen content. Because of the mixture, the ionic bombardment is higher in LF deposition (NHx+ and N2+) than for LF-Free NH3 (only N2+) conditions. This phenomenon produces a more compressive stressed layer in LF than in LF Free NH3 deposition. There is a correlation between the deposition rate and the densification of the layer. If the deposition rate is low, the atomic structure is denser and the porosity is decreased [16,17]. Following Table 2, LF Free NH3 film is denser and the structure is less porous because of its lower hydrogen content and residual compressive stress.
Figures 1 and 2 show that HF sample contains a higher quantity of Si-H bonding whereas LF and LF Free NH3 contain a higher quantity of N-H bonds. Depending on the atomic content of Si and N, hydrogen is contained in Si-H or N-H bonding.
 |
Fig. 1 Absorbance infra-red spectra of LF, HF and LF Free NH3 samples. |
 |
Fig. 2 Quantification of [Si-H], [N-H] and [H] from infra-red spectra of LF, HF and LF Free NH3 samples. |
3.3 The chemical structure influences the moisture resistance
The moisture resistance was evaluated by water vapor and condensed water. The chemical reaction of water with silicon nitride is well-known [3,4,12]. It is described by equations 2 to 5. For water vapor attacks, i.e. 85 °C /85% RH, gaseous water molecules adsorb at the surface of the silicon nitride layer and react to create silicon dioxide and ammonia gas (Eq. (2) and (3)). For condensed water attacks, the silicon dioxide produced from equation 2 is gradually dissolved in water (Eq. (4) and (5)).
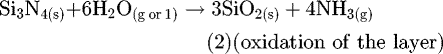 (2) (oxidation of the layer)
(2) (oxidation of the layer)
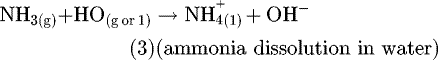 (3) (ammonia dissolution in water)
(3) (ammonia dissolution in water)
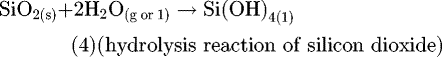 (4) (hydrolysis reaction of silicon dioxide)
(4) (hydrolysis reaction of silicon dioxide)
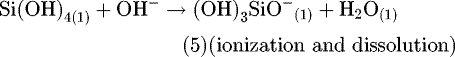 (5) (ionization and dissolution)
(5) (ionization and dissolution)
The robustness of the different layers was evaluated by measuring the silicon oxide thickness and by the dissolution rate of silicon nitride in water.
3.3.1 Water vapor attacks of silicon nitride layers
Figure 3 indicates that silicon dioxide thickness increases with exposure time. At the initial state, about 3–6 nm of SiO2 is formed on samples due to the ambient air oxidization. HF samples in ambient air contain less SiO2 than the other samples. Si-H rich film (HF sample) seems to oxidize slowly at the beginning but after 500 hours, the thickness of SiO2 becomes higher than the other samples. By contrast, the sample with N-H rich content oxidizes quickly, but at the end there is less silicon dioxide formed. The LF Free NH3 samples contain less N-H bonding and the SiO2 thickness remains almost constant. Moreover, in the literature, no case is found where the entire layer of silicon nitride is converted into silicon dioxide with water vapor attack. 10 nm is the maximum thickness of silicon dioxide formed with silicon nitride and water vapor [4,9]. For water vapor attack, once the top of the layer is oxidized, the oxidation process may be limited.
At 1600 hours, the Si-H rich sample (HF) forms about 9.6 nm of silicon dioxide, but this information is insufficient to classify this chemical structure as not robust to moisture because we do not know if this higher SiO2 thickness is related to lower moisture resistance or to the high Si content. An additional test giving direct information about the water resistance of the layer is therefore necessary.
 |
Fig. 3 Silicon oxide thickness as a function of exposure time at 85 °C/85% RH. |
3.3.2 Liquid water attacks
The loss of thickness of silicon nitride increases with the dip time of samples in water at 85 °C (Fig. 4). Moreover, although the composition of the plateau is the same, the analyzed depth decreases when the temperature of the water increases (Fig. 5). For example, HF samples soaked in water at 85 °C for 127 hours have a lost thickness (dissolved SixNyHz) of about 35 nm. This value is an average of the 34 nm determined by SE (Fig. 4) and 36 nm by XPS measurements (Fig. 5). This is consistent with the dissolution reaction. The same results are observed for LF and LF Free NH3 samples. Table 3 reports the dissolution rate and the activation energy of the dissolution reaction from the Arrhenius law (Eq. 1). The HF sample, with a Si-H rich content needs more energy (0.88 eV) to activate the dissolution reaction and the dissolution rate is lower than in the other samples. By contrast, the LF sample with N-H rich content needs less energy (0.67 eV) to dissolve in water and the dissolution rate is faster. The orders of magnitude of the values are consistent with the work of J.W Osenbach and W.R. Knolle [4], for a 1/0.9 Si/N sample. At 100 °C, the dissolution rate in water is 6.3 nm/h for 0.63 eV. These results show a relationship between the chemical structure and the moisture resistance. It can be explained by the chemical mechanism of water attack on silicon nitride (Fig. 6); when the initial structure of the silicon nitride is N-H rich, the water attack starts to the step 3 and less energy is needed to initiate the nucleophilic attacks of water. According to J.N. Chiang et al. [18], the Si2N-H and −H2O+ are isoelectronic and can be easily substituted with each other; this means that during hydrolysis reaction, Si-N-H bonds can be substituted by Si-OH2+ which activates the dissolution process. Consequently, the [N-H] is proportional to the hydrolysis rate. Samples LF and LF Free NH3 are N-H rich content. They are more moisture sensitive and react rapidly with water [19]. The HF sample, on the other hand, is less sensitive to water because Si-H rich film crosses by another chemical mechanism needing more energy to form Si-OH bonds (Fig. 7). According to W-S. Liao and S-C. Lee [10], Si-H film can be used as a passivation layer; the addition of a thin layer of Si-H (30 nm) on the top of SixNyHz layer can protect against air oxidization for 14 months. This affirmation is in agreement with the first point (initial state) shown in Figure 3 where the HF sample contains the least thickness of silicon dioxide.
 |
Fig. 4 Lost thicknesses of LF (red), HF (blue) and LF Free NH3 (green) samples measured by SE as a function of the exposure time of sample in water at 85 °C. |
 |
Fig. 5 Composition of the layer as a function of the analyzed depth obtained before and after dissolution of the HF sample soaked in water at initial state up to 100 °C for different durations. These data are deduced from XPS measurements. |
Arrhenius analysis results for the different deposition conditions at different water temperatures.
 |
Fig. 6 Detailed chemical mechanism between water and Si3N4 from the equation 2 leading to the dissolution of the layer. |
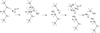 |
Fig. 7 The chemical mechanism between water and SixNyHz silicon rich structure (Si-H rich structure). |
4 Conclusions
By coupling various characterization techniques like SE, XPS and IR, we achieved a better understanding of the relationship between the deposition conditions, the chemical structure and the robustness of silicon nitride to water/moisture. We demonstrate that the kinetic of the chemical reaction between water and silicon nitride depends on hydrogen bonding; for high radio frequency deposition (HF sample-13.56 MHz), there is a moisture resistant silicon-rich (and so Si-H rich) layer, whereas for low radio frequency deposition (LF sample-0.10 MHz), there is a less moisture resistant nitrogen-rich (and so N-H rich) layer. The mixture SiH4/N2 in low radio frequency decreases the hydrogen content and improves moisture resistance. The bonding of N-H in the chemical structure of silicon nitride is moisture sensitive and decreases the water resistance of the layer. It will be interesting to study the effect of mechanical stress on chemical bonding stability and the consequences on the chemical reaction kinetic with water.
Acknowledgement
We express our sincere thanks for the residual mechanical stress measurements made by Patrice Gergaud from the CEA.
References
- M. Vogt, R. Hauptmann, Plasma-deposited passivation layers for moisture and water protection, Surf. Coat. Technol. 74–75, 676–681 (1995), DOI: 10.1016/0257-8972(95)08268-9 [Google Scholar]
- G. Schmitt, J.-W. Schultze, F. Faßbender, G. Buß, H. Lüth, M. Schöning, Passivation and corrosion of microelectrode arrays, Electrochim. Acta. 44, 3865–3883 (1999), DOI: 10.1016/S0013-4686(99)00094-8 [Google Scholar]
- H. Lin, L. Xu, X. Chen, X. Wang, M. Sheng, F. Stubhan, K.-H. Merkel, J. Wilde, Moisture-resistant properties of SiNx films prepared by PECVD, Thin Solid Films 333, 71–76 (1998), DOI: 10.1016/S0040-6090(98)00812-8 [Google Scholar]
- J.W.O. and W.R. Knolle, Behavior of a − SiN: H and a − SiON: H Films in Condensed Water, J. Electrochem. Soc. 139, 3346–3351 (1992) [Google Scholar]
- F.H.P.M. Habraken, A.E.T. Kuiper, Silicon nitride and oxynitride films, Mater. Sci. Eng. R Rep. 12, 123–175 (1994), DOI: 10.1016/0927-796X(94)90006-X [Google Scholar]
- W.a. Lanford, M.J. Rand, The hydrogen content of plasma-deposited silicon nitride, J. Appl. Phys. 49, 2473–2477 (1978), DOI: 10.1063/1.325095 [Google Scholar]
- J. Kanicki, Role of hydrogen in silicon nitride films prepared by various depositions techniques, Mater. Res. Soc. 118, 671–677 (1988), DOI: 10.1557/PROC-118-671 [CrossRef] [Google Scholar]
- V.S. Nguyen, W.A. Lanford, Variation of hydrogen bonding, depth profiles, and spin density in plasma-deposited silicon nitride and oxynitride film with deposition mechanism, J. Electrochem. Soc. 107, 970–974 (1986) [Google Scholar]
- J.A.A. and W.A.P. S. I. Raider, R. Flitsch, Surface oxidation of silicon nitride films, Electrochem. Soc. 123, (1976), DOI: 10.1149/1.2132877 [Google Scholar]
- W.L. and S. Lee, Water-resistant coating on low temperature amorphous silicon nitride films by a thin layer of amorphous silicon hydrogen alloy, J. Electrochem. Soc. 144, 1477–1481 (1997) [Google Scholar]
- S.K. Kang, S.W. Hwang, H. Cheng, S. Yu, B.H. Kim, J.H. Kim, Y. Huang, J.A. Rogers, Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics, Adv. Funct. Mater. 24, 4427–4434 (2014), DOI: 10.1002/adfm.201304293 [Google Scholar]
- E. Laarz, B.V. Zhmud, L. Bergström, Dissolution and deagglomeration of silicon nitride in aqueous medium, J. Am. Ceram. Soc. 83, 2394–2400 (2000), DOI: 10.1111/j.1151-2916.2000.tb01567.x [Google Scholar]
- D.S. Wuu, W.C. Lo, C.C. Chiang, H.B. Lin, L.S. Chang, R.H. Horng, C.L. Huang, Y.J. Gao, Water and oxygen permeation of silicon nitride films prepared by plasma-enhanced chemical vapor deposition, Surf. Coatings Technol. 198, 114–117 (2005), DOI: 10.1016/j.surfcoat.2004.10.034 [CrossRef] [Google Scholar]
- A.M. Andringa, A. Perrotta, K. De Peuter, H.C.M. Knoops, W.M.M. Kessels, M. Creatore, Low-temperature plasma-assisted atomic layer deposition of silicon nitride moisture permeation barrier layers, ACS Appl. Mater. Interfaces. 7, 22525–22532 (2015), DOI: 10.1021/acsami.5b06801 [CrossRef] [Google Scholar]
- W. Liao, C. Lin, S. Lee, Oxidation of silicon nitride prepared by plasma-enhanced chemical vapor deposition at low temperature, Appl. Phys. Lett. 65, 2229–2231 (2001), DOI: 10.1063/1.112772 [Google Scholar]
- R.W.D.H. Dun, P. Pan, F.R. White, Mechanisms of plasma-enhanced silicon nitride deposition using SiH4/N2 mixture, J. Electrochem. Soc. 128, 1555–1563 (n.d.) [Google Scholar]
- T. Karabacak, Y.-P. Zhao, G.-C. Wang, T.-M. Lu, Growth front roughening in silicon nitride films by plasma-enhanced chemical vapor deposition, Phys. Rev. B. 66, 1–10 (2002), DOI: 10.1103/PhysRevB.66.075329 [Google Scholar]
- D.W.H.J.N. Chiang, S.G. Ghanayem, Low-temperature hydrolysis (oxidation) of plasma-deposited silicon nitride films, Chem. Mater. 1, 194–198 (1989), DOI: 10.1021/cm00002a006 [Google Scholar]
- T. Oku, M. Okumura, M. Totsuka, T. Shiga, M. Takemi, Moisture resistance of insulating films for compound semiconductor devices, in: CS MANTECH Conference, Denver, USA 2014, pp. 179–182 [Google Scholar]
Cite this article as: Catheline Cazako, Karim Inal, Alain Burr, Frederic Georgi, Rodolphe Cauro, Hypothetic impact of chemical bonding on the moisture resistance of amorphous SixNyHz by plasma-enhanced chemical vapor deposition, Metall. Res. Technol. 115, 406 (2018)
All Tables
Arrhenius analysis results for the different deposition conditions at different water temperatures.
All Figures
 |
Fig. 1 Absorbance infra-red spectra of LF, HF and LF Free NH3 samples. |
| In the text | |
 |
Fig. 2 Quantification of [Si-H], [N-H] and [H] from infra-red spectra of LF, HF and LF Free NH3 samples. |
| In the text | |
 |
Fig. 3 Silicon oxide thickness as a function of exposure time at 85 °C/85% RH. |
| In the text | |
 |
Fig. 4 Lost thicknesses of LF (red), HF (blue) and LF Free NH3 (green) samples measured by SE as a function of the exposure time of sample in water at 85 °C. |
| In the text | |
 |
Fig. 5 Composition of the layer as a function of the analyzed depth obtained before and after dissolution of the HF sample soaked in water at initial state up to 100 °C for different durations. These data are deduced from XPS measurements. |
| In the text | |
 |
Fig. 6 Detailed chemical mechanism between water and Si3N4 from the equation 2 leading to the dissolution of the layer. |
| In the text | |
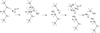 |
Fig. 7 The chemical mechanism between water and SixNyHz silicon rich structure (Si-H rich structure). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


