| Issue |
Metall. Res. Technol.
Volume 117, Number 4, 2020
|
|
|---|---|---|
| Article Number | 408 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/metal/2020040 | |
| Published online | 29 July 2020 | |
Regular Article
Evolution of non-metallic inclusions during heat treatment
1
The State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology,
Wuhan
430081, PR China
2
Department of Materials Science and Engineering, Carnegie Mellon University,
Pittsburgh, PA
15213, USA
* e-mail: liuchengsong@wust.edu.cn
Received:
22
February
2020
Accepted:
18
June
2020
Isothermal heat treatment can not only modify steel microstructure, but also non-metallic inclusions. In this work, heat treatment experiments were conducted between 1373 and 1573 K (1100 and 1300 °C) to study the evolution of inclusion composition, morphology, and size distribution. Results showed that during the heat treatment at 1473 and 1573 K (1200 and 1300 °C), two main kinds of inclusions initially in the steel, CaS and MgO–Al2O3–CaO–CaS, gradually transformed to (Ca, Mn)S and MgO–Al2O3–(Ca, Mn)S inclusions, and some MgO–Al2O3–CaO inclusions also transformed to MgO–Al2O3–(Ca, Mn)S. At the lowest temperature studied, 1373 K (1100 °C), little change was observed. No significant changes in number density and area fraction of the measured inclusions were observed, while the average size of inclusions increased after the heat treatment. The extent of transformation of CaS, MgO–Al2O3–CaO–CaS and MgO–Al2O3–CaO inclusions increased with decreasing inclusion size and higher temperature.
Key words: Non-metallic inclusions / heat treatment / transformation / interfacial reactions
© EDP Sciences, 2020
1 Introduction
Uncontrolled non-metallic inclusions in steel may have detrimental effects on mechanical properties such as strength, toughness, and ductility [1–6]. Generally, characteristics of the inclusions are modified and optimized by refining slag, calcium treatment or rare earth treatment in molten steel to minimize their harmfulness and eliminate nozzle clogging during casting [7–13]. However, the final inclusions in steel product are not exactly the same as those in the molten steel. Apart from reactions that occur during solidification [14–18], heat treatment processes that modify steel microstructures also can contribute to the transformation of the inclusions [19–23]. The effects of inclusion formation during solidification and the changes during heat treatment must be examined for a complete understanding of how inclusions evolve to their state in the final product. This work focuses on the effects of solid-state transformations occurring during heat treatment.
There have been several studies that investigated these kinds of transformations, though many fewer than those having investigated inclusion evolution in the liquid state. H. Shibata et al. [24] reported that at a low Si concentration in stainless steel, MnO–SiO2 type inclusions gradually changed into MnO–Cr2O3 type inclusions during heat treatment at 1473 K (1200 °C), while at a high Si content, the MnO–SiO2 type inclusion was stable after heat treatment. Y. Ren et al. [25] reported similar findings, with Cr in 304 stainless steel reacting with manganese silicates to form chromite spinels during heat treatment in the range of 1273 to 1473 K (1000 to 1200 °C). A model was developed in that study assuming the rate of transformation was dependent on diffusion of species in the steel to the inclusion, therefore the transformation rate depended strongly on both temperature and inclusion size. K.H. Kim et al. [26] found the equilibrium oxide with Fe–Mn–Si alloy was not a binary compound of MnO and SiO2 but a ternary compound of MnO, SiO2, and FeO. After heat treatment at 1673 and 1473 K (1400 and 1200 °C), diffusion of oxygen from the oxide to the alloy resulted in new MnO–SiO2 oxide particles precipitating in the alloy near the interface. W. Choi et al. [27] studied the behavior of non-metallic inclusions in Al–Ti deoxidized steels at 1473 K (1200 °C) with three different steels and argued that Al and Ti contents in the steels were critical for the transformation occurrence of Al2O3, Al2O3–TiOx and TiOx inclusions to Fe–Al–O, Fe–Al–Ti–O and Fe–Ti–O inclusions during heat treatment. X.J. Shao et al. [28] observed that during the continuous heating process, large-sized MnS inclusions in non-quenched and tempered steel underwent spheroidization and coarsening processes. In linepipe steels [29], calcium aluminates were found to transform to CaS–Al2O3 during heat treatment at 1473 K (1200 °C), with a kinetic analysis based on solid-state diffusion. In previous work by one of the authors [30,31], solid-state reactions between Fe–Al–Ca alloy and Al2O3–CaO–FeO oxide during heat treatment at 1473 K (1200 °C) were studied. Heat treatment led to the decrease of Al content and increase of Ca content in the alloy. Some Al2O3 particles and CaO · Al2O3 dendritic inclusions precipitated as reaction products in the alloy near the alloy-oxide interface.
Previous studies have confirmed that inclusion characteristics can be modified by heat treatment when all phases are in the solid state. The steels studied here contained 50 ppm S, were Al-killed, and were slightly over-treated with Ca, resulting in excess CaS. However, characteristic transformation of inclusions in the steel during subsequent heat treatment is still unknown, which is essential for hot rolling process and control of steel product quality. In this work, isothermal heat treatment has been carried out at different temperatures in the range of 1373 to 1573 K (1100 to 1300 °C) under an argon atmosphere, and the resulting changes in morphology, composition, size distribution of the inclusions were subsequently analyzed by scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS) using an ASPEX Explorer instrument. At relatively higher heat treatment temperatures (1473 and 1573 K (1200 and 1300 °C)), the two main kinds of inclusions initially in the steels, CaS and MgO–Al2O3–CaO–CaS, gradually transformed to (Ca, Mn)S and MgO–Al2O3–(Ca, Mn)S inclusions, respectively, and some MgO–Al2O3–CaO inclusions also transformed to MgO–Al2O3–(Ca, Mn)S, while little evidence of transformation was observed at 1373 K (1100°C). The extent of transformation increased with decreasing inclusion size and higher temperature.
2 Experimental method
Steel samples were initially taken from the tundish during industrial production. The steel chemical composition listed in Table 1 was measured by inductively coupled plasma mass spectrometry (ICP-MS), combustion analysis (for S) and inert gas fusion (for O). Ca, Mg, and O bound in the inclusions were quantified from inclusion analysis and considered as the total Ca, Mg, and O in the steel, since the dissolved Ca, Mg, and O concentrations were extremely low. Values for Ca, Mg, and O were estimated based on the composition and amount of the inclusions measured by SEM/EDS with ASPEX Explorer instrument. The area fraction of inclusions was assumed to be volume fraction. The densities of steel and inclusion were assumed to be 7500 and 3500 kg · m−3, respectively. The total Ca, Mg and O contents can be calculated by the following equations based on the measured average inclusion compositions.
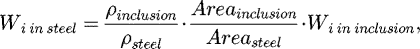 (1)
where, Wi in steel represents the total estimated content of element i in the steel, ρinclusion and ρsteel are the densities of the inclusion and the steel, Areainclusion and Areasteel are the total measured areas of the inclusions and steel, respectively, Wi in inclusion represents the measured average content of element i in the inclusions. Each sample was machined into a cube approximately 10 × 10 × 10 mm. Those steel samples were heat treated at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C), respectively, for 3 h. The heating and cooling rates were 4 K·min−1. Flowing Ar gas was used during the heating, isothermal hold, and cooling periods. A schematic of the setup is shown in Figure 1.
(1)
where, Wi in steel represents the total estimated content of element i in the steel, ρinclusion and ρsteel are the densities of the inclusion and the steel, Areainclusion and Areasteel are the total measured areas of the inclusions and steel, respectively, Wi in inclusion represents the measured average content of element i in the inclusions. Each sample was machined into a cube approximately 10 × 10 × 10 mm. Those steel samples were heat treated at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C), respectively, for 3 h. The heating and cooling rates were 4 K·min−1. Flowing Ar gas was used during the heating, isothermal hold, and cooling periods. A schematic of the setup is shown in Figure 1.
The steel samples after heat treatment were mounted in epoxy resin, ground with 320, 800, 1200 and 2000 grit SiC papers, and then mirror polished with a 1 µm diamond suspension. The chemical composition and size distributions of inclusions were measured on metallographically polished samples by SEM/EDS with a Thermo-Fisher/FEI/ASPEX Explorer instrument, operated at an acceleration voltage of 10 kV with 5–7% brightness and 100% contrast. The spatial resolution of the microscope using backscattered electron imaging was measured to be approximately 0.4 µm under those conditions. The area for analysis was at least 21 mm2 for each sample to acquire sufficient data. During microanalysis, the electron beam was rastered over the total exposed area of each inclusion to determine its average composition. Equilibrium thermodynamic calculations were performed with FactSage v. 7.2. The FToxid database was used for the slag, sulfide, and spinel oxide solutions. FactPS was used for the pure oxide inclusions and FSstel was used for the steel solid and liquid phases.
Chemical composition of the steel samples for the heat treatment experiments (values for Mg, Ca, and O were estimated from the inclusion composition and amounts).
 |
Fig. 1 Schematic of apparatus setup for heating experiment. |
3 Results
Figure 2 shows the size distributions of total inclusions in the steel samples before and after heat treatment at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C). Inclusion sizes in all cases mainly located in the range of 0 to 3 µm. Almost no inclusions with diameters greater than 10 µm were found in these steel samples. The size distributions and total numbers of inclusions did not change noticeably during heat treatment, especially for the ones whose diameters larger than 2 µm.
Figure 3 shows the evolution of average composition, number density, average diameter, and area fraction of inclusions in steel samples before and after heat treatment at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C). The average composition of inclusions in steel samples changed significantly as the heat treatment temperature increased, as shown in Figure 3a; the contents of Mn and Ca increased and decreased, respectively, while those of Al, Mg and S relatively remained stable. The number density, average diameter, and area fraction of inclusions in the steel before and after heat treatment at different temperatures are shown in Figure 3b through 3d. There are no significant changes in number density and area fraction of the measured inclusions after the heating, which demonstrates that during the heating process, good Ar gas protection was achieved and there is no obvious oxidation of the steel. However, the average size of inclusions increased after the heating, which may be related to the transformation of the inclusions. When the steels were heated at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C), interfacial reactions between the inclusions and steels would occur, which resulted in changes of average inclusion composition and diameter.
Figure 4 shows the inclusion compositions (mole fraction) on the Mg–Al–Ca, S–Ca–Mn and S–Al–Ca ternary diagrams from tundish samples and after three hours of heat treatment at the temperatures of 1373, 1473, and 1573 K (1100, 1200, and 1300 °C). In the Figure 4 plots, the symbol size is proportional to the number fraction of inclusions of the indicated composition, and the area inside dashed line refers to a region at 1873 K (1600 °C) where the fraction of liquid phase inclusions should be at least 0.5.
As shown in Figures 4a and 4b, the main inclusions from the tundish sample were complex MgO–Al2O3–CaO–CaS and pure CaS. Since there is no S element in the ternary diagram of Ca–Al–Mg, standalone CaS inclusions mainly locate around the Ca component, as shown in Figures 4a, 4d, 4g and 4j. Figure 4c shows that besides MgO–Al2O3–CaO–CaS and pure CaS inclusions, some complex MgO–Al2O3–CaO inclusions without S also formed in the tundish sample. Comparison of Figures 4a and 4b with 4d and 4e shows that little change was observed after heat treatment at 1373 K (1100 °C). However, as shown in Figure 4f, it is noted that almost no MgO–Al2O3–CaO inclusions were observed in the sample after heating at 1373 K (1100 °C). After heat treatment at the higher temperatures, MgO content decreased and Al2O3 content increased in the complex MgO–Al2O3–CaO–CaS inclusions. Tighter distribution of the inclusions shown in the Mg–Al–Ca ternary diagram at higher heat treatment temperature was obtained, as shown in Figures 4g and 4j.
Also after heat treatment at 1473 and 1573 K (1200 and 1300 °C), a more noteworthy phenomenon was that Mn contents in the CaS and complex MgO–Al2O3–CaO–CaS inclusions, shown in Figures 4h and 4k, significantly increased. In the S–Al–Ca ternary diagram shown in Figures 4i and 4l, the molar ratio of sulfur to calcium in the inclusions increased as the MnS content of the inclusions increased after heat treatment.
Figure 5 shows the change in the average composition (mass %) of inclusions with various apparent diameters in the steels in tundish and after the heat treatment. The average composition of inclusions with various inclusion sizes was obtained by equation (2):
 (2)
where javg is the average composition of inclusions in a certain size range, jk and Areak indicate the composition and apparent area of a single inclusion k, n is the inclusion number in the certain size range. In this study, the apparent diameters of the inclusions ranged from 0 ∼ 1 to 6 ∼ 10 µm. Experimental results indicated that after heat treatment, the inclusions with smaller apparent diameters contained higher Mn and S contents as well as lower Ca content.
(2)
where javg is the average composition of inclusions in a certain size range, jk and Areak indicate the composition and apparent area of a single inclusion k, n is the inclusion number in the certain size range. In this study, the apparent diameters of the inclusions ranged from 0 ∼ 1 to 6 ∼ 10 µm. Experimental results indicated that after heat treatment, the inclusions with smaller apparent diameters contained higher Mn and S contents as well as lower Ca content.
According to Figure 4, standalone CaS and complex oxide-sulfide inclusions were present in the liquid steel. Figure 6 shows the morphology and measured element distribution of a standalone CaS inclusion before and after heat treatment at 1473 K (1200 °C).
Transformations of complex MgO–Al2O3–CaO and MgO–Al2O3–CaO–CaS inclusions also were observed after heat treatment. A typical oxide + sulfide inclusion from the tundish sample is shown in Figure 7a. Spherical oxide inclusions were also observed as shown in Figure 7b. Figure 7c shows a typical oxide + sulfide inclusion after heat treatment. No standalone MgO–Al2O3–CaO oxide inclusions were observed after heat treatment.
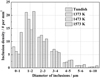 |
Fig. 2 Size distributions of total inclusions in the steels before and after the heat treatment at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C). |
 |
Fig. 3 Evolution of (a) average composition; (b) number density; (c) average diameter; and (d) area fraction of inclusions in steel samples before and after heat treatment at different temperatures. |
 |
Fig. 4 Chemical compositions (mole fraction) of inclusions in tundish and after heat treatments. In each diagram the symbol size is proportional to the number fraction of inclusions with that composition. When indicated, the dashed line represents the region in which 50% of the inclusions would be liquid according to the equilibrium phase diagram. |
 |
Fig. 5 Change in the average composition (mass %) of inclusions with various apparent sizes in the steels (a) in tundish and after heat treatment at (b) 1373 K (1100 °C); (c) 1473 K (1200 °C) and; (d) 1573 K (1300 °C). |
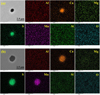 |
Fig. 6 Morphology and measured element distribution of typical inclusions in the steels: (a) CaS in the tundish sample; (b) (Ca, Mn)S in the sample after heat treatment at 1473 K (1200 °C). |
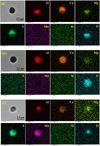 |
Fig. 7 Morphology and measured element distribution of typical inclusions in the steels: (a) MgO–Al2O3–CaO–CaS in the tundish sample; (b) MgO–Al2O3–CaO in the tundish sample; and (c) MgO–Al2O3–(Ca, Mn)S in the sample after heat treatment at 1473 K (1200 °C). |
4 Discussion
In this study, two main kinds of initial standalone inclusions were found in the tundish sample, CaS and MgO–Al2O3–CaO–CaS inclusions, as well as some spherical MgO–Al2O3–CaO inclusions. After the heat treatment at 1373, 1473 and 1573 K (1100, 1200 and 1300 °C), typical oxide + sulfide inclusions and sulfide inclusions were observed in steels, without standalone MgO–Al2O3–CaO oxide inclusions. Although the size distribution and total number of inclusions did not change noticeably during heat treatment, with higher heating temperature, Mn content increased and Ca content decreased in the inclusions, especially for those smaller ones. This indicated more extensive transformation of smaller CaS, MgO–Al2O3–CaO–CaS and MgO–Al2O3–CaO inclusions. However, those phenomena were restrained during the heating at 1373 K (1100 °C).
Predicted equilibrium inclusion phases in the steel with the temperature were calculated by FactSage 7.2, as shown in Figure 8. Calculations in Figure 8a indicated that MgO–Al2O3–CaO liquid oxide and solid CaS inclusions would be stable in the liquid steel. A single sulfide solid solution (Ca, Mn)S formed at high temperature, but a miscibility gap occurred in the Ca–Mn–S system at approximately 1573 K (1300 °C) [32–34]. Figures 8b and 8c show the change of component amounts in the two sulfide phases (MS-c#1 and MS-c#2) as a function of temperature.
A comparison of predicted equilibrium inclusion compositions and measured compositions is given in Figure 9. It was clear that reaction with the steel occurred, even though the equilibrium compositions were not reached. In addition, as the heating temperature increased, especially at 1473 and 1573 K (1200 and 1300 °C), the measured inclusion compositions gradually became close to the predicted equilibrium compositions. Further support for reaction with the steel can be seen in Figure 5, which showed more extensive composition changes for smaller inclusions. This was expected because supply of species like Mn and S should be limited by diffusion in the steel, so the flux should be larger as particle size decreased.
As noted in Figures 6 and 7, there were three different inclusion types observed in the tundish sample: standalone sulfides (CaS), oxide-sulfide (MgO–Al2O3–CaO–CaS), and standalone oxides (MgO–Al2O3–CaO). Figure 10 shows the transformation of each type schematically. The standalone sulfide inclusions transformed to (Ca, Mn)S solutions due to the reaction with [Mn] and [S] in the steel. The evolution of the oxide + sulfide inclusions was similar, with the initially CaS part of the inclusions reacting with Mn and S in the steel. This led to the oxide + (Ca, Mn)S inclusions shown in Figure 7c.
Some of the oxide inclusions initially contained no sulfide phase, but after heat treatment no standalone oxide inclusions were observed. Other work has observed transformation of the CaO component to CaS after heat treatment via the reaction (3) [14,29,35–38]. It was thought that during the heating at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C) for 3 h, the reaction between [Al] and CaO firstly occurred, resulting in an increase of Al2O3 in inclusions, and then the reaction between [S] and CaO occurred leading to an increase of CaS content in the inclusions, although this reaction was commonly observed in molten steel and during steel solidification.
 (3)
(3)
Then dissolved [Mn] would react with CaS and lead to the formation of complex MgO–Al2O3–(Ca, Mn)S inclusions [39], as shown in Figures 7b and 7c. The sequence of chemistry changes should be MgO–Al2O3–CaO → MgO–Al2O3–CaO–CaS → MgO–Al2O3–(Ca, Mn)S. Morphology of the (Ca, Mn)S phase in the MgO–Al2O3–(Ca, Mn)S inclusions also shows angular shape. It was also noted that the morphology of the inclusion became angular after the heat treatment.
 |
Fig. 8 Predicted equilibrium inclusion phases in the steel with the temperature: (a) predicted equilibrium inclusion phases; component amount in the (b) MS-c#1 phase and (c) MS-c#2 phase. |
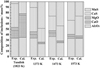 |
Fig. 9 Comparisons of experimental results and predicted equilibrium inclusion compositions before and after the heat treatment. |
 |
Fig. 10 Schematic of the transformation mechanism of (a) CaS; (b) MgO–Al2O3–CaO–CaS and; (c) MgO–Al2O3–CaO inclusions during the heat treatment of steels. |
5 Conclusions
In the present study, heat treatment experiments have been conducted to study the transformation mechanism of inclusions in the steels at different temperatures in the range of 1373 to 1573 K (1100 to 1300 °C). The conclusions are as follows:
During the heat treatment at 1473 and 1573 K (1200 and 1300 °C), two main kinds of inclusions in the steels, CaS and MgO–Al2O3–CaO–CaS inclusions, gradually transformed to (Ca, Mn)S and MgO–Al2O3–(Ca, Mn)S inclusions, respectively, and some MgO–Al2O3–CaO inclusions also transformed to MgO–Al2O3–(Ca, Mn)S inclusions, while those phenomena were restrained during the heating at 1373 K (1100 °C).
No significant changes in number density and area fraction of the measured inclusions were observed, while the average size of inclusions increased after the heat treatment.
The transformation extents of CaS, MgO–Al2O3–CaO–CaS and MgO–Al2O3–CaO inclusions generally have a negative correlation with inclusion size, and a positive correlation with heat treatment temperature, in the range of 1373 to 1573 K (1100 to 1300 °C). With higher heating temperature, the measured inclusion compositions gradually became closer to the predicted equilibrium compositions.
Acknowledgment
The authors acknowledge support from the member companies of the Center for Iron and Steelmaking Research and the International Postdoctoral Exchange Fellowship Program (2017) by China Postdoctoral Council as well as use of the Materials Characterization Facility at Carnegie Mellon, supported by grant MCF-677785.
References
- N.G. Kolbasnikov, M.A. Matveev, P.A. Mishnev, Effect of structure factor on high-temperature ductility of pipe steels, Met. Sci. Heat Treat. 58(1-2), 51–57 (2016) [CrossRef] [Google Scholar]
- B.T. Lu, J.L. Luo, Crack initiation and early propagation of X70 steel in simulated near-neutral pH groundwater, Corrosion 62(8), 723–731 (2006) [CrossRef] [Google Scholar]
- T. Hara, H. Asahi, H. Ogawa. Conditions of hydrogen-induced corrosion occurrence of X65 grade line pipe steels in sour environments, Corrosion 60(12), 1113–1121 (2004) [CrossRef] [Google Scholar]
- B. Beidokhti, A. Dolati, A.H. Koukabi. Effects of alloying elements and microstructure on the susceptibility of the welded HSLA steel to hydrogen-induced cracking and sulfide stress cracking, Mater. Sci. Eng. A. 507(1-2), 167–173 (2009) [CrossRef] [Google Scholar]
- A. Takahashi, H. Ogawa. Influence of microhardness and inclusion on stress oriented hydrogen induced cracking of line pipe steels, ISIJ Int. 36(3), 334–340 (1996) [CrossRef] [Google Scholar]
- Y.F. Sui, C.S. Yue, B. Peng, et al., Optimization of slag chemistry toward inclusion control for 28CrMo47 drill pipe steel based on viscosity and equilibration studies, Steel Res. Int. 87(6), 752–760 (2015) [CrossRef] [Google Scholar]
- N. Verma, P.C. Pistorius, R.J. Fruehan, et al., Transient inclusion evolution during modification of alumina inclusions by calcium in liquid steel: Part I. Background, experimental techniques and analysis methods, Metall. Mater. Trans. B. 42(4), 711–719 (2011) [CrossRef] [Google Scholar]
- M.M. Song, B. Song, S.H. Zhang, et al., Effect of heat input on microstructure and toughness of rare earth-contained C–Mn steel, J. Iron Steel Res. Int. 25(10), 1033–1042 (2018) [CrossRef] [Google Scholar]
- H. Zhang, C.S. Liu, Q. Lin, et al., Formation of plastic inclusions in U71Mnk high-speed heavy-rail steel refined by CaO-SiO2-Al2O3-MgO slag, Metall. Mater. Trans. B. 50(1), 459–470 (2019) [CrossRef] [Google Scholar]
- L.Z. Wang, S.F. Yang, J.S. Li, et al., Improving cleanliness of 95CrMo drill rod steel by slag refining, Metall. Mater. Trans. B. 47(1), 99–107 (2016) [CrossRef] [Google Scholar]
- H.Y. Mu, T.S. Zhang, R.J. Fruehan, et al., Reduction of CaO and MgO slag components by Al in liquid Fe, Metall. Mater. Trans. B. 49(4), 1665–1674 (2018) [CrossRef] [Google Scholar]
- Y. Ren, Y.F. Wang, S.S. Li, et al., Detection of non-metallic inclusions in steel continuous casting billets, Metall. Mater. Trans. B. 45(4), 1291–1303 (2014) [CrossRef] [Google Scholar]
- J.J. Wang, W.F. Li, Y. Ren, et al., Thermodynamic and kinetic analysis for transformation of oxide inclusions in solid 304 stainless steels, Steel Res. Int. 90(7), 1800600 (2019) [CrossRef] [Google Scholar]
- Y. Wang, M. Valdez, S. Sridhar Formation of CaS on Al2O3–CaO inclusions during solidification of steels, Metall. Mater. Trans. B. 33(4), 625–632 (2002) [CrossRef] [Google Scholar]
- S.K. Choudhary, A. Ghosh Mathematical model for prediction of composition of inclusions formed during solidification of liquid steel, ISIJ Int. 49(12), 1819–1827 (2009) [CrossRef] [Google Scholar]
- S.Y. Chen, X.D. Yue, G.C. Jin, et al., Behavior of inclusions in process of solid growth during solidification of Fe-0.15C-0.8Mn steel, J. Iron Steel Res. Int. 19(5), 17 (2012) [CrossRef] [Google Scholar]
- M. Suzuki, R. Yamaguchi, K. Murakami, et al., Inclusion particle growth during solidification of stainless steel, ISIJ Int. 41(3), 247–256 (2001) [CrossRef] [EDP Sciences] [Google Scholar]
- W. Chen, Y. Ren, L.F. Zhang, Large eddy simulation on the fluid flow, solidification and entrapment of inclusions in the steel along the full continuous casting slab strand, JOM. 70(12), 2968–2979 (2018) [CrossRef] [Google Scholar]
- I. Takahashi, T. Sakae, T. Yoshida, Changes of the nonmetallic inclusion by heating, Tetsu-to-Hagané 53(3), 350–352 (1967) [CrossRef] [Google Scholar]
- H. Shibata, T. Tanaka, K. Kimura, et al., Composition change in oxide inclusions of stainless steel by heat treatment, Ironmak. Steelmak. 37(7), 522–528 (2010) [CrossRef] [Google Scholar]
- C.S. Liu, H.W. Ni, S.F. Yang, et al., Interfacial reaction mechanism between multi-component oxides and solid alloys deoxidized by Mn and Si during heat treatment, Ironmak. Steelmak. 45(3), 195–203 (2018) [CrossRef] [Google Scholar]
- W. Yang, C.B. Guo, C. Li, et al., Transformation of inclusions in pipeline steels during solidification and cooling, Metall. Mater. Trans. B. 48(5), 2267–2273 (2017) [CrossRef] [Google Scholar]
- Y. Wang, W. Yang, L.F. Zhang, Effect of cooling rate on oxide inclusions during solidification of 304 stainless steel, Steel Res. Int. 90(7), 1900027 (2019) [CrossRef] [Google Scholar]
- H. Shibata, K. Kimura, T. Tanaka, et al., Mechanism of change in chemical composition of oxide inclusions in Fe–Cr alloys deoxidized with Mn and Si by heat treatment at 1473 K, ISIJ Int. 51(12), 1944–1950 (2011) [CrossRef] [Google Scholar]
- Y. Ren, L.F. Zhang, P.C. Pistorius, Transformation of oxide inclusions in type 304 stainless steels during heat treatment, Metall. Mater. Trans. B. 48(5), 2281–2292 (2017) [CrossRef] [Google Scholar]
- K.H. Kim, S.J. Kim, H. Shibata, et al., Reaction between MnO–SiO2–FeO oxide and Fe–Mn–Si solid alloy during heat treatment, ISIJ Int. 54(10), 2144–2153 (2014) [CrossRef] [Google Scholar]
- W. Choi, H. Matsuura, F. Tsukihashi, Changing behavior of non-metallic inclusions in solid iron deoxidized by Al-Ti addition during heating at 1473 K, ISIJ Int. 51(12), 1951–1956 (2011) [CrossRef] [Google Scholar]
- X.J. Shao, X.H. Wang, M. Jiang, et al., Effect of heat treatment conditions on shape control of large-sized elongated MnS inclusions in resulfurized free-cutting steels, ISIJ Int. 51(12), 1995–2001 (2011) [CrossRef] [Google Scholar]
- Y.P. Chu, W.F. Li, Y. Ren, et al., Transformation of inclusions in linepipe steels during heat treatment, Metall. Mater. Trans. B. 50(4), 2047–2062 (2019) [CrossRef] [Google Scholar]
- C.S. Liu, S.F. Yang, J.S. Li, et al., Solid-state reaction between Fe–Al–Ca alloy and Al2O3–CaO–FeO oxide during heat treatment at 1473 K (1200 °C), Metall. Mater. Trans. B. 48(2), 1348–1357 (2017) [CrossRef] [Google Scholar]
- C.S. Liu, S.F. Yang, J.S. Li, et al., The influence of FeO on the reaction between Fe–Al–Ca alloy and Al2O3–CaO–FeO oxide during heat treatment at 1473 K, Metals 7(4), 129 (2017) [Google Scholar]
- R. Kiessling, C. Westman, The MnS-CaS system and its metallurgical significance, J. Iron Steel Inst. 208(7), 699–700 (1970) [Google Scholar]
- B.J. Skinner, F.D. Luce, Solid solutions of the type (Ca, Mg, Mn, Fe)S and their use as geothermometers for the enstatite chondrites, Am. Miner. 56(7-8), 1269–1276 (1971) [Google Scholar]
- C.H. Leung, L.H.V. Vlack, Solubility limits in binary (Ca, Mn) Chalcogenides, J. Am. Ceram. Soc. 62(11-12), 613–621 (1979) [Google Scholar]
- D.Z. Lu, G.A. Irons, W.K. Lu, Calculation of CaS and MnS activities and their application to calcium treatment of steel, Ironmak. Steelmak. 18(5), 342–346 (1991) [Google Scholar]
- G. Xu, Z.H. Jiang, Y. Li, Formation mechanism of CaS-bearing inclusions and the rolling deformation in Al-killed, low-alloy steel with Ca treatment, Metall. Mater. Trans. B. 47(4), 2411–2420 (2016) [CrossRef] [Google Scholar]
- J.F. Xu, F.X. Huang, X.H. Wang, Formation mechanism of CaS–Al2O3 inclusions in low sulfur Al-killed steel after calcium treatment, Metall. Mater. Trans. B. 47(2), 1217–1227 (2016) [CrossRef] [Google Scholar]
- W. Yang, L.F. Zhang, X.H. Wang, et al., Characteristics of inclusions in low carbon Al-killed steel during ladle furnace refining and calcium treatment, ISIJ Int. 53(8), 1401–1410 (2013) [CrossRef] [Google Scholar]
- R.X. Piao, H.G. Lee, Y.B. Kang, Activity measurement of the CaS–MnS sulfide solid solution and thermodynamic modeling of the CaO–MnO–Al2O3–CaS–MnS–Al2S3 system, ISIJ Int. 53(12), 2132–2141 (2013) [CrossRef] [Google Scholar]
Cite this article as: Chengsong Liu, Bryan Webler, Evolution of non-metallic inclusions during heat treatment, Metall. Res. Technol. 117, 408 (2020)
All Tables
Chemical composition of the steel samples for the heat treatment experiments (values for Mg, Ca, and O were estimated from the inclusion composition and amounts).
All Figures
 |
Fig. 1 Schematic of apparatus setup for heating experiment. |
| In the text | |
 |
Fig. 2 Size distributions of total inclusions in the steels before and after the heat treatment at 1373, 1473, and 1573 K (1100, 1200, and 1300 °C). |
| In the text | |
 |
Fig. 3 Evolution of (a) average composition; (b) number density; (c) average diameter; and (d) area fraction of inclusions in steel samples before and after heat treatment at different temperatures. |
| In the text | |
 |
Fig. 4 Chemical compositions (mole fraction) of inclusions in tundish and after heat treatments. In each diagram the symbol size is proportional to the number fraction of inclusions with that composition. When indicated, the dashed line represents the region in which 50% of the inclusions would be liquid according to the equilibrium phase diagram. |
| In the text | |
 |
Fig. 5 Change in the average composition (mass %) of inclusions with various apparent sizes in the steels (a) in tundish and after heat treatment at (b) 1373 K (1100 °C); (c) 1473 K (1200 °C) and; (d) 1573 K (1300 °C). |
| In the text | |
 |
Fig. 6 Morphology and measured element distribution of typical inclusions in the steels: (a) CaS in the tundish sample; (b) (Ca, Mn)S in the sample after heat treatment at 1473 K (1200 °C). |
| In the text | |
 |
Fig. 7 Morphology and measured element distribution of typical inclusions in the steels: (a) MgO–Al2O3–CaO–CaS in the tundish sample; (b) MgO–Al2O3–CaO in the tundish sample; and (c) MgO–Al2O3–(Ca, Mn)S in the sample after heat treatment at 1473 K (1200 °C). |
| In the text | |
 |
Fig. 8 Predicted equilibrium inclusion phases in the steel with the temperature: (a) predicted equilibrium inclusion phases; component amount in the (b) MS-c#1 phase and (c) MS-c#2 phase. |
| In the text | |
 |
Fig. 9 Comparisons of experimental results and predicted equilibrium inclusion compositions before and after the heat treatment. |
| In the text | |
 |
Fig. 10 Schematic of the transformation mechanism of (a) CaS; (b) MgO–Al2O3–CaO–CaS and; (c) MgO–Al2O3–CaO inclusions during the heat treatment of steels. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


